Role of PPAR-delta in the development of zymosan-induced multiple organ failure: an experiment mice study
- PMID: 20167109
- PMCID: PMC2844385
- DOI: 10.1186/1476-9255-7-12
Role of PPAR-delta in the development of zymosan-induced multiple organ failure: an experiment mice study
Abstract
Background: Peroxisome proliferator-activated receptor (PPAR)-beta/delta is a nuclear receptor transcription factor that regulates gene expression in many important biological processes. It is expressed ubiquitously, especially white adipose tissue, heart, muscle, intestine, placenta and macrophages but many of its functions are unknown. Saturated and polyunsaturated fatty acids activate PPAR-beta/delta, but physiological ligands have not yet been identified. In the present study, we investigated the anti-inflammatory effects of PPAR-beta/delta activation, through the use of GW0742 (0,3 mg/kg 10% Dimethyl sulfoxide (DMSO) i.p), a synthetic high affinity ligand, on the development of zymosan-induced multiple organ failure (MOF).
Methods: Multiple organ failure (MOF) was induced in mice by administration of zymosan (given at 500 mg/kg, i.p. as a suspension in saline). The control groups were treated with vehicle (0.25 ml/mouse saline), while the pharmacological treatment was the administration of GW0742 (0,3 mg/kg 10% DMSO i.p. 1 h and 6 h after zymosan administration). MOF and systemic inflammation in mice was assessed 18 hours after administration of zymosan.
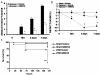
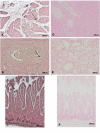
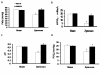
Results: Treatment with GW0742 caused a significant reduction of the peritoneal exudate formation and of the neutrophil infiltration caused by zymosan resulting in a reduction in myeloperoxidase activity. The PPAR-beta/delta agonist, GW0742, at the dose of 0,3 mg/kg in 10% DMSO, also attenuated the multiple organ dysfunction syndrome caused by zymosan. In pancreas, lung and gut, immunohistochemical analysis of some end points of the inflammatory response, such as inducible nitric oxide synthase (iNOS), nitrotyrosine, poly (ADP-ribose) (PAR), TNF- and IL-1as well as FasL, Bax, Bcl-2 and apoptosis, revealed positive staining in sections of tissue obtained from zymosan-injected mice. On the contrary, these parameters were markedly reduced in samples obtained from mice treated with GW0742
Conclusions: In this study, we have shown that GW0742 attenuates the degree of zymosan-induced non-septic shock in mice.
Figures














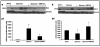



Similar articles
-
PD98059, a specific MAP kinase inhibitor, attenuates multiple organ dysfunction syndrome/failure (MODS) induced by zymosan in mice.Pharmacol Res. 2010 Feb;61(2):175-87. doi: 10.1016/j.phrs.2009.09.008. Epub 2009 Oct 9. Pharmacol Res. 2010. PMID: 19819333
-
Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces the development of nonseptic shock induced by zymosan in mice.Crit Care Med. 2004 Feb;32(2):457-66. doi: 10.1097/01.CCM.0000109446.38675.61. Crit Care Med. 2004. PMID: 14758164
-
Evidence for the role of peroxisome proliferator-activated receptor-beta/delta in the development of spinal cord injury.J Pharmacol Exp Ther. 2010 May;333(2):465-77. doi: 10.1124/jpet.110.165605. Epub 2010 Feb 22. J Pharmacol Exp Ther. 2010. PMID: 20176685
-
Absence of peroxisome proliferators-activated receptors (PPAR)alpha enhanced the multiple organ failure induced by zymosan.Shock. 2006 Nov;26(5):477-84. doi: 10.1097/01.shk.0000230299.78515.2c. Shock. 2006. PMID: 17047518
-
Inducible nitric oxide synthase knockout mice exhibit resistance to the multiple organ failure induced by zymosan.Shock. 2001 Jul;16(1):51-8. doi: 10.1097/00024382-200116010-00010. Shock. 2001. PMID: 11442316
Cited by
-
Effects of the PPAR-β/δ agonist GW0742 during resuscitated porcine septic shock.Intensive Care Med Exp. 2013 Dec;1(1):28. doi: 10.1186/2197-425X-1-9. Epub 2013 Oct 29. Intensive Care Med Exp. 2013. PMID: 26266797 Free PMC article.
-
Regulation of satellite cell function in sarcopenia.Front Aging Neurosci. 2014 Sep 22;6:246. doi: 10.3389/fnagi.2014.00246. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25295003 Free PMC article. Review.
-
Delayed activation of PPAR-β/δ improves long-term survival in mouse sepsis: effects on organ inflammation and coagulation.Innate Immun. 2018 May;24(4):262-273. doi: 10.1177/1753425918771748. Epub 2018 Apr 26. Innate Immun. 2018. PMID: 29697010 Free PMC article.
-
Anti-Inflammatory Activity of Fucan from Spatoglossum schröederi in a Murine Model of Generalized Inflammation Induced by Zymosan.Mar Drugs. 2023 Oct 26;21(11):557. doi: 10.3390/md21110557. Mar Drugs. 2023. PMID: 37999381 Free PMC article.
-
Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast, and lung carcinogenesis.Cancer Metastasis Rev. 2011 Dec;30(3-4):619-40. doi: 10.1007/s10555-011-9320-1. Cancer Metastasis Rev. 2011. PMID: 22037942 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

