R-Ras regulates beta1-integrin trafficking via effects on membrane ruffling and endocytosis
- PMID: 20167113
- PMCID: PMC2830936
- DOI: 10.1186/1471-2121-11-14
R-Ras regulates beta1-integrin trafficking via effects on membrane ruffling and endocytosis
Abstract
Background: Integrin-mediated cell adhesion and spreading is dramatically enhanced by activation of the small GTPase, R-Ras. Moreover, R-Ras localizes to the leading edge of migrating cells, and regulates membrane protrusion. The exact mechanisms by which R-Ras regulates integrin function are not fully known. Nor is much known about the spatiotemporal relationship between these two molecules, an understanding of which may provide insight into R-Ras regulation of integrins.
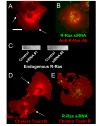
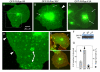
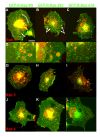
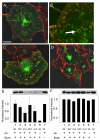
Results: GFP-R-Ras localized to the plasma membrane, most specifically in membrane ruffles, in Cos-7 cells. GFP-R-Ras was endocytosed from these ruffles, and trafficked via multiple pathways, one of which involved large, acidic vesicles that were positive for Rab11. Cells transfected with a dominant negative form of GFP-R-Ras did not form ruffles, had decreased cell spreading, and contained numerous, non-trafficking small vesicles. Conversely, cells transfected with the constitutively active form of GFP-R-Ras contained a greater number of ruffles and large vesicles compared to wild-type transfected cells. Ruffle formation was inhibited by knock-down of endogenous R-Ras with siRNA, suggesting that activated R-Ras is not just a component of, but also an architect of ruffle formation. Importantly, beta1-integrin co-localized with endogenous R-Ras in ruffles and endocytosed vesicles. Expression of dominant negative R-Ras or knock down of R-Ras by siRNA prevented integrin accumulation into ruffles, impaired endocytosis of beta1-integrin, and decreased beta1-integrin-mediated adhesion. Knock-down of R-Ras also perturbed the dynamics of another membrane-localized protein, GFP-VSVG, suggesting a more global role for R-Ras on membrane dynamics. However, while R-Ras co-internalized with integrins, it did not traffic with VSVG, which instead moved laterally out of ruffles within the plane of the membrane, suggesting multiple levels of regulation of and by R-Ras.
Conclusions: Our results suggest that integrin function involves integrin trafficking via a cycle of membrane protrusion, ruffling, and endocytosis regulated by R-Ras, providing a novel mechanism by which integrins are linked to R-Ras through control of membrane dynamics.
Figures


Similar articles
-
Palmitoylation regulates vesicular trafficking of R-Ras to membrane ruffles and effects on ruffling and cell spreading.Small GTPases. 2012 Jul-Sep;3(3):139-53. doi: 10.4161/sgtp.21084. Epub 2012 Jul 1. Small GTPases. 2012. PMID: 22751447 Free PMC article.
-
The small GTPase R-Ras regulates organization of actin and drives membrane protrusions through the activity of PLCepsilon.J Cell Sci. 2006 Apr 1;119(Pt 7):1307-19. doi: 10.1242/jcs.02835. Epub 2006 Mar 14. J Cell Sci. 2006. PMID: 16537651
-
The R-Ras/RIN2/Rab5 complex controls endothelial cell adhesion and morphogenesis via active integrin endocytosis and Rac signaling.Cell Res. 2012 Oct;22(10):1479-501. doi: 10.1038/cr.2012.110. Epub 2012 Jul 24. Cell Res. 2012. PMID: 22825554 Free PMC article.
-
Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling.Cell Signal. 2009 Jan;21(1):1-6. doi: 10.1016/j.cellsig.2008.06.020. Epub 2008 Jul 3. Cell Signal. 2009. PMID: 18647649 Free PMC article. Review.
-
Mechanisms of integrin activation and trafficking.Curr Opin Cell Biol. 2011 Oct;23(5):607-14. doi: 10.1016/j.ceb.2011.08.005. Epub 2011 Sep 14. Curr Opin Cell Biol. 2011. PMID: 21924601 Review.
Cited by
-
Depalmitoylation and cell physiology: APT1 as a mediator of metabolic signals.Am J Physiol Cell Physiol. 2024 Apr 1;326(4):C1034-C1041. doi: 10.1152/ajpcell.00542.2023. Epub 2024 Feb 12. Am J Physiol Cell Physiol. 2024. PMID: 38344800 Free PMC article. Review.
-
Gene expression profiles of the NCI-60 human tumor cell lines define molecular interaction networks governing cell migration processes.PLoS One. 2012;7(5):e35716. doi: 10.1371/journal.pone.0035716. Epub 2012 May 3. PLoS One. 2012. PMID: 22570691 Free PMC article.
-
The leucine-rich region of Flightless I interacts with R-ras to regulate cell extension formation.Mol Biol Cell. 2018 Oct 1;29(20):2481-2493. doi: 10.1091/mbc.E18-03-0147. Epub 2018 Aug 9. Mol Biol Cell. 2018. PMID: 30091651 Free PMC article.
-
Reciprocal discoidin domain receptor signaling strengthens integrin adhesion to connect adjacent tissues.bioRxiv [Preprint]. 2023 May 16:2023.03.14.532639. doi: 10.1101/2023.03.14.532639. bioRxiv. 2023. Update in: Elife. 2023 Jul 05;12:RP87037. doi: 10.7554/eLife.87037. PMID: 36993349 Free PMC article. Updated. Preprint.
-
EndophilinAs regulate endosomal sorting of BDNF-TrkB to mediate survival signaling in hippocampal neurons.Sci Rep. 2017 May 19;7(1):2149. doi: 10.1038/s41598-017-02202-4. Sci Rep. 2017. PMID: 28526875 Free PMC article.
References
-
- Saez R, Chan AM, Miki T, Aaronson SA. Oncogenic activation of human R-ras by point mutations analogous to those of prototype H-ras oncogenes. Oncogene. 1994;9:2977–2982. - PubMed
-
- Cox AD, Brtva TR, Lowe DG, Der CJ. R-Ras induces malignant, but not morphologic, transformation of NIH3T3 cells. Oncogene. 1994;9:3281–3288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

