The ClpP protease homologue is required for the transmission traits and cell division of the pathogen Legionella pneumophila
- PMID: 20167127
- PMCID: PMC2838875
- DOI: 10.1186/1471-2180-10-54
The ClpP protease homologue is required for the transmission traits and cell division of the pathogen Legionella pneumophila
Abstract
Background: Legionella pneumophila, the intracellular bacterial pathogen that causes Legionnaires' disease, exhibit characteristic transmission traits such as elevated stress tolerance, shortened length and virulence during the transition from the replication phase to the transmission phase. ClpP, the catalytic core of the Clp proteolytic complex, is widely involved in many cellular processes via the regulation of intracellular protein quality.
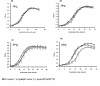
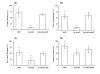
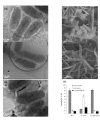
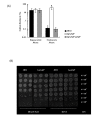
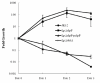
Results: In this study, we showed that ClpP was required for optimal growth of L. pneumophila at high temperatures and under several other stress conditions. We also observed that cells devoid of clpP exhibited cell elongation, incomplete cell division and compromised colony formation. Furthermore, we found that the clpP-deleted mutant was more resistant to sodium stress and failed to proliferate in the amoebae host Acanthamoeba castellanii.
Conclusions: The data present in this study illustrate that the ClpP protease homologue plays an important role in the expression of transmission traits and cell division of L. pneumophila, and further suggest a putative role of ClpP in virulence regulation.
Figures

Similar articles
-
ClpP-deletion impairs the virulence of Legionella pneumophila and the optimal translocation of effector proteins.BMC Microbiol. 2016 Aug 2;16(1):174. doi: 10.1186/s12866-016-0790-8. BMC Microbiol. 2016. PMID: 27484084 Free PMC article.
-
The amoebae plate test implicates a paralogue of lpxB in the interaction of Legionella pneumophila with Acanthamoeba castellanii.Microbiology (Reading). 2005 Jan;151(Pt 1):167-182. doi: 10.1099/mic.0.27563-0. Microbiology (Reading). 2005. PMID: 15632436
-
New Global Insights on the Regulation of the Biphasic Life Cycle and Virulence Via ClpP-Dependent Proteolysis in Legionella pneumophila.Mol Cell Proteomics. 2022 May;21(5):100233. doi: 10.1016/j.mcpro.2022.100233. Epub 2022 Apr 12. Mol Cell Proteomics. 2022. PMID: 35427813 Free PMC article.
-
Legionella pneumophila adaptation to intracellular life and the host response: clues from genomics and transcriptomics.FEBS Lett. 2007 Jun 19;581(15):2829-38. doi: 10.1016/j.febslet.2007.05.026. Epub 2007 May 21. FEBS Lett. 2007. PMID: 17531986 Review.
-
Legionella pneumophila: an aquatic microbe goes astray.FEMS Microbiol Rev. 2002 Jun;26(2):149-62. doi: 10.1111/j.1574-6976.2002.tb00607.x. FEMS Microbiol Rev. 2002. PMID: 12069880 Review.
Cited by
-
Phenotypic and genomic characterization of a Vibrio parahaemolyticus strain causing disease in Penaeus vannamei provides insights into its niche adaptation and pathogenic mechanism.Microb Genom. 2021 May;7(5):000549. doi: 10.1099/mgen.0.000549. Microb Genom. 2021. PMID: 33952389 Free PMC article.
-
The Temporal Expression of Global Regulator Protein CsrA Is Dually Regulated by ClpP During the Biphasic Life Cycle of Legionella pneumophila.Front Microbiol. 2019 Nov 7;10:2495. doi: 10.3389/fmicb.2019.02495. eCollection 2019. Front Microbiol. 2019. PMID: 31787938 Free PMC article.
-
Exploring anti-bacterial compounds against intracellular Legionella.PLoS One. 2013 Sep 13;8(9):e74813. doi: 10.1371/journal.pone.0074813. eCollection 2013. PLoS One. 2013. PMID: 24058631 Free PMC article.
-
Development of heat-shock resistance in Legionella pneumophila modeled by experimental evolution.Appl Environ Microbiol. 2023 Sep 28;89(9):e0066623. doi: 10.1128/aem.00666-23. Epub 2023 Sep 5. Appl Environ Microbiol. 2023. PMID: 37668382 Free PMC article.
-
Trans-translation is essential in the human pathogen Legionella pneumophila.Sci Rep. 2016 Nov 28;6:37935. doi: 10.1038/srep37935. Sci Rep. 2016. PMID: 27892503 Free PMC article.
References
-
- Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, Sharrar RG, Harris J, Mallison GF, Martin SM, McDade JE, Shepard CC, Brachman PS. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. - PubMed
-
- Kaufmann AF, McDade JE, Patton CM, Bennett JV, Skaliy P, Feeley JC, Anderson DC, Potter ME, Newhouse VF, Gregg MB, Brachman PS. Pontiac fever: isolation of the etiologic agent (Legionella pneumophilia) and demonstration of its mode of transmission. Am J Epidemiol. 1981;114:337–347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

