A novel fed-batch based cultivation method provides high cell-density and improves yield of soluble recombinant proteins in shaken cultures
- PMID: 20167131
- PMCID: PMC2841585
- DOI: 10.1186/1475-2859-9-11
A novel fed-batch based cultivation method provides high cell-density and improves yield of soluble recombinant proteins in shaken cultures
Abstract
Background: Cultivations for recombinant protein production in shake flasks should provide high cell densities, high protein productivity per cell and good protein quality. The methods described in laboratory handbooks often fail to reach these goals due to oxygen depletion, lack of pH control and the necessity to use low induction cell densities. In this article we describe the impact of a novel enzymatically controlled fed-batch cultivation technology on recombinant protein production in Escherichia coli in simple shaken cultures.
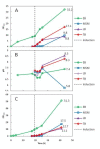
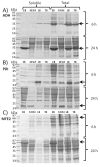
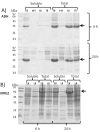
Results: The enzymatic glucose release system together with a well-balanced combination of mineral salts and complex medium additives provided high cell densities, high protein yields and a considerably improved proportion of soluble proteins in harvested cells. The cultivation method consists of three steps: 1) controlled growth by glucose-limited fed-batch to OD600 approximately 10, 2) addition of growth boosters together with an inducer providing efficient protein synthesis within a 3 to 6 hours period, and 3) a slow growth period (16 to 21 hours) during which the recombinant protein is slowly synthesized and folded. Cell densities corresponding to 10 to 15 g l(-1) cell dry weight could be achieved with the developed technique. In comparison to standard cultures in LB, Terrific Broth and mineral salt medium, we typically achieved over 10-fold higher volumetric yields of soluble recombinant proteins.
Conclusions: We have demonstrated that by applying the novel EnBase Flo cultivation system in shaken cultures high cell densities can be obtained without impairing the productivity per cell. Especially the yield of soluble (correctly folded) proteins was significantly improved in comparison to commonly used LB, Terrific Broth or mineral salt media. This improvement is thought to result from a well controlled physiological state during the whole process. The higher volumetric yields enable the use of lower culture volumes and can thus significantly reduce the amount of time and effort needed for downstream processing or process optimization. We claim that the new cultivation system is widely applicable and, as it is very simple to apply, could widely replace standard shake flask approaches.
Figures

Similar articles
-
High Yield of Recombinant Protein in Shaken E. coli Cultures with Enzymatic Glucose Release Medium EnPresso B.Methods Mol Biol. 2017;1586:127-137. doi: 10.1007/978-1-4939-6887-9_8. Methods Mol Biol. 2017. PMID: 28470602
-
High cell density cultivation and recombinant protein production with Escherichia coli in a rocking-motion-type bioreactor.Microb Cell Fact. 2010 May 30;9:42. doi: 10.1186/1475-2859-9-42. Microb Cell Fact. 2010. PMID: 20509968 Free PMC article.
-
The fed-batch principle for the molecular biology lab: controlled nutrient diets in ready-made media improve production of recombinant proteins in Escherichia coli.Microb Cell Fact. 2016 Jun 17;15(1):110. doi: 10.1186/s12934-016-0513-8. Microb Cell Fact. 2016. PMID: 27317421 Free PMC article. Review.
-
Enzyme controlled glucose auto-delivery for high cell density cultivations in microplates and shake flasks.Microb Cell Fact. 2008 Nov 18;7:31. doi: 10.1186/1475-2859-7-31. Microb Cell Fact. 2008. PMID: 19017379 Free PMC article.
-
Recombinant protein expression in high cell density fed-batch cultures of Escherichia coli.Biotechnology (N Y). 1992 Dec;10(12):1550-6. doi: 10.1038/nbt1292-1550. Biotechnology (N Y). 1992. PMID: 1369204 Review.
Cited by
-
Design of experiments-based high-throughput strategy for development and optimization of efficient cell disruption protocols.Eng Life Sci. 2016 Oct 5;17(11):1166-1172. doi: 10.1002/elsc.201600030. eCollection 2017 Nov. Eng Life Sci. 2016. PMID: 32624744 Free PMC article.
-
Fed-batch operation in special microtiter plates: a new method for screening under production conditions.J Ind Microbiol Biotechnol. 2014 Mar;41(3):513-25. doi: 10.1007/s10295-013-1396-x. Epub 2014 Jan 14. J Ind Microbiol Biotechnol. 2014. PMID: 24419608
-
High-level fed-batch fermentative expression of an engineered Staphylococcal protein A based ligand in E. coli: purification and characterization.AMB Express. 2015 Dec;5(1):70. doi: 10.1186/s13568-015-0155-y. Epub 2015 Nov 10. AMB Express. 2015. PMID: 26556030 Free PMC article.
-
Optimization of Culture Conditions for Oxygen-Tolerant Regulatory [NiFe]-Hydrogenase Production from Ralstonia eutropha H16 in Escherichia coli.Microorganisms. 2021 May 31;9(6):1195. doi: 10.3390/microorganisms9061195. Microorganisms. 2021. PMID: 34073092 Free PMC article.
-
Efficient Antibody Assembly in E. coli Periplasm by Disulfide Bond Folding Factor Co-expression and Culture Optimization.Appl Biochem Biotechnol. 2017 Oct;183(2):520-529. doi: 10.1007/s12010-017-2502-8. Epub 2017 May 10. Appl Biochem Biotechnol. 2017. PMID: 28488120 Free PMC article.
References
-
- Sambrook J, Russell D. Molecular cloning:a laboratory manual. 3. CHL Press; 2001.
-
- Overton TW, Griffiths L, Patel MD, Hobman JL, Penn CW, Cole JA. Microarray analysis of gene regulation by oxygen, nitrate, nitrite, FNR, NarL and NarP during anaerobic growth of Escherichia coli: new insights into microbial physiology. Biochem Soc Trans. 2006;34:104–107. doi: 10.1042/BST0340104. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

