Dominance of highly divergent feline leukemia virus A progeny variants in a cat with recurrent viremia and fatal lymphoma
- PMID: 20167134
- PMCID: PMC2837606
- DOI: 10.1186/1742-4690-7-14
Dominance of highly divergent feline leukemia virus A progeny variants in a cat with recurrent viremia and fatal lymphoma
Abstract
Background: In a cat that had ostensibly recovered from feline leukemia virus (FeLV) infection, we observed the reappearance of the virus and the development of fatal lymphoma 8.5 years after the initial experimental exposure to FeLV-A/Glasgow-1. The goals of the present study were to investigate this FeLV reoccurrence and molecularly characterize the progeny viruses.
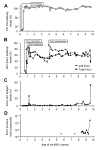
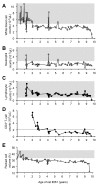
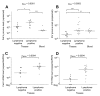
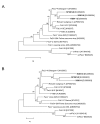
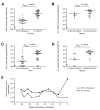
Results: The FeLV reoccurrence was detected by the presence of FeLV antigen and RNA in the blood and saliva. The cat was feline immunodeficiency virus positive and showed CD4+ T-cell depletion, severe leukopenia, anemia and a multicentric monoclonal B-cell lymphoma. FeLV-A, but not -B or -C, was detectable. Sequencing of the envelope gene revealed three FeLV variants that were highly divergent from the virus that was originally inoculated (89-91% identity to FeLV-A/Glasgow-1). In the long terminal repeat 31 point mutations, some previously described in cats with lymphomas, were detected. The FeLV variant tissue provirus and viral RNA loads were significantly higher than the FeLV-A/Glasgow-1 loads. Moreover, the variant loads were significantly higher in lymphoma positive compared to lymphoma negative tissues. An increase in the variant provirus blood load was observed at the time of FeLV reoccurrence.
Conclusions: Our results demonstrate that ostensibly recovered FeLV provirus-positive cats may act as a source of infection following FeLV reactivation. The virus variants that had largely replaced the inoculation strain had unusually heavily mutated envelopes. The mutations may have led to increased viral fitness and/or changed the mutagenic characteristics of the virus.
Figures
Similar articles
-
Long-term follow up of feline leukemia virus infection and characterization of viral RNA loads using molecular methods in tissues of cats with different infection outcomes.Virus Res. 2015 Feb 2;197:137-50. doi: 10.1016/j.virusres.2014.12.025. Epub 2014 Dec 30. Virus Res. 2015. PMID: 25553598
-
Reduced Folate Carrier: an Entry Receptor for a Novel Feline Leukemia Virus Variant.J Virol. 2019 Jun 14;93(13):e00269-19. doi: 10.1128/JVI.00269-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30996094 Free PMC article.
-
Evaluation of the effect of short-term treatment with the integrase inhibitor raltegravir (Isentress) on the course of progressive feline leukemia virus infection.Vet Microbiol. 2015 Feb 25;175(2-4):167-78. doi: 10.1016/j.vetmic.2014.10.031. Epub 2014 Nov 3. Vet Microbiol. 2015. PMID: 25500005
-
How molecular methods change our views of FeLV infection and vaccination.Vet Immunol Immunopathol. 2008 May 15;123(1-2):119-23. doi: 10.1016/j.vetimm.2008.01.017. Epub 2008 Jan 19. Vet Immunol Immunopathol. 2008. PMID: 18295346 Review.
-
Real-time PCR investigation of feline leukemia virus proviral and viral RNA loads in leukocyte subsets.Vet Immunol Immunopathol. 2008 May 15;123(1-2):124-8. doi: 10.1016/j.vetimm.2008.01.018. Epub 2008 Jan 19. Vet Immunol Immunopathol. 2008. PMID: 18304650 Review.
Cited by
-
Xenotransfusion of Blood from Dog to Cat: Should Canine Blood Be Our First Choice for Feline Transfusion in Emergency Situations?Vet Sci. 2022 Feb 28;9(3):106. doi: 10.3390/vetsci9030106. Vet Sci. 2022. PMID: 35324834 Free PMC article.
-
Prevalence, Geographic Distribution, Risk Factors and Co-Infections of Feline Gammaherpesvirus Infections in Domestic Cats in Switzerland.Viruses. 2019 Aug 6;11(8):721. doi: 10.3390/v11080721. Viruses. 2019. PMID: 31390829 Free PMC article.
-
Retroviral DNA--the silent winner: blood transfusion containing latent feline leukemia provirus causes infection and disease in naïve recipient cats.Retrovirology. 2015 Dec 21;12:105. doi: 10.1186/s12977-015-0231-z. Retrovirology. 2015. PMID: 26689419 Free PMC article.
-
Feline leukaemia virus infection: A practical approach to diagnosis.J Feline Med Surg. 2020 Sep;22(9):831-846. doi: 10.1177/1098612X20941785. J Feline Med Surg. 2020. PMID: 32845225 Free PMC article. Review.
-
2020 AAFP Feline Retrovirus Testing and Management Guidelines.J Feline Med Surg. 2020 Jan;22(1):5-30. doi: 10.1177/1098612X19895940. J Feline Med Surg. 2020. PMID: 31916872 Free PMC article.
References
-
- Yamamoto JK, Sparger E, Ho EW, Andersen PR, OConner TP, Mandell CP, Lowenstinde L, Munn R, Pedersen NC. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–1258. - PubMed
-
- Hoover EA, Mullins JI. Feline leukemia virus infection and diseases. J Am Vet Med Assoc. . 1991;199(10):1287–1297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

