Alarm characterization for continuous glucose monitors used as adjuncts to self-monitoring of blood glucose
- PMID: 20167166
- PMCID: PMC2825623
- DOI: 10.1177/193229681000400106
Alarm characterization for continuous glucose monitors used as adjuncts to self-monitoring of blood glucose
Abstract
Background: Continuous glucose monitoring (CGM) devices available in the United States are approved for use as adjuncts to self-monitoring of blood glucose (SMBG). Alarm evaluation in the Clinical and Laboratory Standards Institute (CLSI) guideline for CGM does not specifically address devices that employ both CGM and SMBG. In this report, an alarm evaluation method is proposed for these devices.
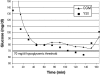
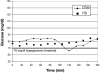
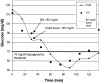
Method: The proposed method builds on the CLSI method using data from an in-clinic study of subjects with type 1 diabetes. CGM was used to detect glycemic events, and SMBG was used to determine treatment. To optimize detection of a single glucose level, such as 70 mg/dl, a range of alarm threshold settings was evaluated. The alarm characterization provides a choice of alarm settings that trade off detection and false alarms. Detection of a range of high glucose levels was similarly evaluated.
Results: Using low glucose alarms, detection of 70 mg/dl within 30 minutes increased from 64 to 97% as alarm settings increased from 70 to 100 mg/dl, and alarms that did not require treatment (SMBG >85 mg/dl) increased from 18 to 52%. Using high glucose alarms, detection of 180 mg/dl within 30 minutes increased from 87 to 96% as alarm settings decreased from 180 to 165 mg/dl, and alarms that did not require treatment (SMBG <180 mg/dl) increased from 24 to 42%.
Conclusion: The proposed alarm evaluation method provides information for choosing appropriate alarm thresholds and reflects the clinical utility of CGM alarms.
2010 Diabetes Technology Society.
Figures
References
-
- Pitzer KR, Desai S, Dunn T, Edelman S, Jayalakshmi Y, Kennedy J, Tamada JA, Potts RO. Detection of hypoglycemia with the GlucoWatch Biographer. Diabetes Care. 2001;24(5):803–804. - PubMed
-
- Clinical and Laboratory Standards Institute. Performance metrics for continuous interstitial glucose monitoring; approved guideline. 2008. POCT05-A (28)33.
-
- International Standards Organization. In vitro diagnostic test systems: requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. 2003. ISO 15197:2003(E)
-
- Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125–1130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical