A review of standards and statistics used to describe blood glucose monitor performance
- PMID: 20167170
- PMCID: PMC2825627
- DOI: 10.1177/193229681000400110
A review of standards and statistics used to describe blood glucose monitor performance
Abstract
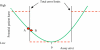
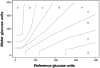
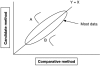
Glucose performance is reviewed in the context of total error, which includes error from all sources, not just analytical. Many standards require less than 100% of results to be within specific tolerance limits. Analytical error represents the difference between tested glucose and reference method glucose. Medical errors include analytical errors whose magnitude is great enough to likely result in patient harm. The 95% requirements of International Organization for Standardization 15197 and others make little sense, as up to 5% of results can be medically unacceptable. The current American Diabetes Association standard lacks a specification for user error. Error grids can meaningfully specify allowable glucose error. Infrequently, glucose meters do not provide a glucose result; such an occurrence can be devastating when associated with a life-threatening event. Nonreporting failures are ignored by standards. Estimates of analytical error can be classified into the four following categories: imprecision, random patient interferences, protocol-independent bias, and protocol-dependent bias. Methods to estimate total error are parametric, nonparametric, modeling, or direct. The Westgard method underestimates total error by failing to account for random patient interferences. Lawton's method is a more complete model. Bland-Altman, mountain plots, and error grids are direct methods and are easier to use as they do not require modeling. Three types of protocols can be used to estimate glucose errors: method comparison, special studies and risk management, and monitoring performance of meters in the field. Current standards for glucose meter performance are inadequate. The level of performance required in regulatory standards should be based on clinical needs but can only deal with currently achievable performance. Clinical standards state what is needed, whether it can be achieved or not. Rational regulatory decisions about glucose monitors should be based on robust statistical analyses of performance.
2010 Diabetes Technology Society.
Figures

References
-
- Westgard JO, Carey RN, Wold S. Criteria for judging precision and accuracy in method development and evaluation. Clin Chem. 1974;20(7):825–833. - PubMed
-
- Krouwer JS. Recommendation to treat continuous variable errors like attribute errors. Clin Chem Lab Med. 2006;44(7):797–798. - PubMed
-
- ADA 1987 American Diabetes Association. Consensus statement on self monitoring of blood glucose. Diabetes Care. 1987;10(1):93–99. - PubMed
-
- In vitro diagnostic test systems–requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. Geneva, Switzerland: International Organization for Standardization; 2003. ISO 15197.
-
- CLSI/NCCLS . CLSI/NCCLS document C30-A2. Wayne, PA: NCCLS; 2002. Point-of-care blood glucose testing in acute and chronic care facilities approved guideline.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

