Interferon-induced Sus scrofa Mx1 blocks endocytic traffic of incoming influenza A virus particles
- PMID: 20167191
- PMCID: PMC2826089
- DOI: 10.1051/vetres/2010001
Interferon-induced Sus scrofa Mx1 blocks endocytic traffic of incoming influenza A virus particles
Abstract
The interferon-induced Mx proteins of vertebrates are dynamin-like GTPases, some isoforms of which can additionally inhibit the life cycle of certain RNA viruses. Here we show that the porcine Mx1 protein (poMx1) inhibits replication of influenza A virus and we attempt to identify the step at which the viral life cycle is blocked. In infected cells expressing poMx1, the level of transcripts encoding the viral nucleoprotein is significantly lower than normal, even when secondary transcription is prevented by exposure to cycloheximide. This reveals that a pretranscriptional block participates to the anti-influenza activity. Binding and internalization of incoming virus particles are normal in the presence of poMx1 but centripetal traffic to the late endosomes is interrupted. Surprisingly but decisively, poMx1 significantly alters binding of early endosome autoantigen 1 to early endosomes and/or early endosome size and spatial distribution. This is compatible with impairment of traffic of the endocytic vesicles to the late endosomes.
INRA, EDP Sciences, 2010.
Figures

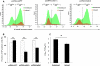
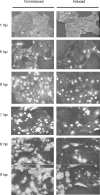

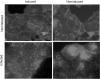
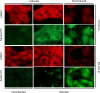

Similar articles
-
Mx proteins: GTPases involved in the interferon-induced antiviral state.Ciba Found Symp. 1993;176:233-43; discussion 243-7. doi: 10.1002/9780470514450.ch15. Ciba Found Symp. 1993. PMID: 7507812 Review.
-
Porcine Mx1 Protein Inhibits Classical Swine Fever Virus Replication by Targeting Nonstructural Protein NS5B.J Virol. 2018 Mar 14;92(7):e02147-17. doi: 10.1128/JVI.02147-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343573 Free PMC article.
-
Differential anti-influenza activity among allelic variants at the Sus scrofa Mx1 locus.J Interferon Cytokine Res. 2007 Feb;27(2):147-55. doi: 10.1089/jir.2006.0119. J Interferon Cytokine Res. 2007. PMID: 17316142
-
In vitro inhibition of vesicular stomatitis virus replication by purified porcine Mx1 protein fused to HIV-1 Tat protein transduction domain (PTD).Antiviral Res. 2013 Aug;99(2):149-57. doi: 10.1016/j.antiviral.2013.05.009. Epub 2013 May 28. Antiviral Res. 2013. PMID: 23727591
-
The Mx GTPase family of interferon-induced antiviral proteins.Microbes Infect. 2007 Nov-Dec;9(14-15):1636-43. doi: 10.1016/j.micinf.2007.09.010. Epub 2007 Sep 14. Microbes Infect. 2007. PMID: 18062906 Review.
Cited by
-
Effects of the polymorphisms of Mx1, BAT2 and CXCL12 genes on immunological traits in pigs.Mol Biol Rep. 2012 Mar;39(3):2417-27. doi: 10.1007/s11033-011-0992-y. Epub 2011 Jun 12. Mol Biol Rep. 2012. PMID: 21667240
-
Transcriptional profiles of PBMCs from pigs infected with three genetically diverse porcine reproductive and respiratory syndrome virus strains.Mol Biol Rep. 2018 Oct;45(5):675-688. doi: 10.1007/s11033-018-4204-x. Epub 2018 Jun 7. Mol Biol Rep. 2018. PMID: 29882085 Free PMC article.
-
Association of multi-pathogenic infections with BAT2, CXCL12, Mx1 and EHMT2 variations in pigs.Mol Biol Rep. 2012 Aug;39(8):8169-76. doi: 10.1007/s11033-012-1664-2. Epub 2012 Apr 25. Mol Biol Rep. 2012. PMID: 22531939
-
GTPase activity of porcine Mx1 plays a dominant role in inhibiting the N-Nsp9 interaction and thus inhibiting PRRSV replication.J Virol. 2024 Apr 16;98(4):e0184423. doi: 10.1128/jvi.01844-23. Epub 2024 Mar 4. J Virol. 2024. PMID: 38436247 Free PMC article.
-
Mammalian and Avian Host Cell Influenza A Restriction Factors.Viruses. 2021 Mar 22;13(3):522. doi: 10.3390/v13030522. Viruses. 2021. PMID: 33810083 Free PMC article. Review.
References
-
- Apodaca G., Endocytic traffic in polarized epithelial cells: role of actin and microtubule cytoskeleton, Traffic (2001) 2:149–159 - PubMed
-
- Baise E., Pire G., Leroy M., Gérardin J., Goris N., De Clercq K., et al., Conditional expression of type I interferon-induced bovine Mx1 GTPase in a stable transgenic vero cell line interferes with replication of vesicular stomatitis virus, J. Interferon Cytokine Res. (2004) 24:513–521 - PubMed
-
- Barret T., Wolstenholme A.J., Maby B.W.J., Transcription and replication of influenza virus RNA, Virology (1979) 98:211–225 - PubMed
-
- Bucci C., Parton R.G., Mather I.H., Stunnenberg H., Simons K., Hoflacks B., Zerial M., The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway, Cell (1992) 70:715–728 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
