Calorimetric and spectroscopic studies of aminoglycoside binding to AT-rich DNA triple helices
- PMID: 20167243
- PMCID: PMC3977217
- DOI: 10.1016/j.biochi.2010.02.004
Calorimetric and spectroscopic studies of aminoglycoside binding to AT-rich DNA triple helices
Abstract
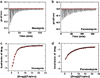
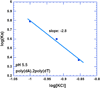
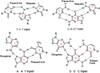
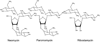
Calorimetric and fluorescence techniques were used to characterize the binding of aminoglycosides-neomycin, paromomycin, and ribostamycin, with 5'-dA(12)-x-dT(12)-x-dT(12)-3' intramolecular DNA triplex (x = hexaethylene glycol) and poly(dA).2poly(dT) triplex. Our results demonstrate the following features: (1) UV thermal analysis reveals that the T(m) for triplex decreases with increasing pH value in the presence of neomycin, while the T(m) for the duplex remains unchanged. (2) The binding affinity of neomycin decreases with increased pH, although there is an increase in observed binding enthalpy. (3) ITC studies conducted in two buffers (sodium cacodylate and MOPS) yield the number of protonated drug amino groups (Deltan) as 0.29 and 0.40 for neomycin and paromomycin interaction with 5'-dA(12)-x-dT(12)-x-dT(12)-3', respectively. (4) The specific heat capacity change (DeltaC(p)) determined by ITC studies is negative, with more negative values at lower salt concentrations. From 100 mM to 250 mM KCl, the DeltaC(p) ranges from -402 to -60 cal/(mol K) for neomycin. At pH 5.5, a more positive DeltaC(p) is observed, with a value of -98 cal/(mol K) at 100 mM KCl. DeltaC(p) is not significantly affected by ionic strength. (5) Salt dependence studies reveal that there are at least three amino groups of neomycin participating in the electrostatic interactions with the triplex. (6) FID studies using thiazole orange were used to derive the AC(50) (aminoglycoside concentration needed to displace 50% of the dye from the triplex) values. Neomycin shows a seven fold higher affinity than paromomycin and eleven fold higher affinity than ribostamycin at pH 6.8. (7) Modeling studies, consistent with UV and ITC results, show the importance of an additional positive charge in triplex recognition by neomycin. The modeling and thermodynamic studies indicate that neomycin binding to the DNA triplex depends upon significant contributions from charge as well as shape complementarity of the drug to the DNA triplex Watson-Hoogsteen groove.
Copyright 2010 Elsevier Masson SAS. All rights reserved.
Figures


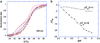




References
-
- Maher LJ. Prospects for the therapeutic use of antigene oligonucleotides. Cancer Invest. 1996;14:66–82. - PubMed
-
- Giovannangeli C, Helene C. Progress in developments of triplex-based strategies antisense. Nucleic Acid Drug Dev. 1997;7:413–421. - PubMed
-
- Praseuth D, Guieysse-Peugeot A, Helene C. Triple helix formation and the antigene strategy for sequence-specific control of gene expression. Biochem. Biophys. Res. Commun. 1999;1489:181–206. - PubMed
-
- Mirkin SM, Lyamichev VI, Drushlyak KN, Dobrynin VN, Filippov SA, Frank-Kamenetskii M. DNA H form reuires a homopurine-homopyrimidine mirror repeat. Nature. 1987;330:495–497. - PubMed
-
- Maher LJI, Wold B, Dervan PB. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989;245:725–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

