Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6
- PMID: 20167600
- PMCID: PMC2857083
- DOI: 10.1074/jbc.M109.058669
Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6
Abstract
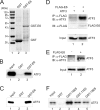
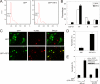
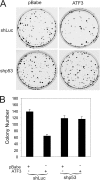
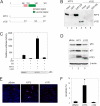
Genomic integration of human papillomavirus (HPV) DNA accounts for more than 90% of cervical cancers. High-risk genital HPVs encode E6 proteins that can interact with a cellular ubiquitin ligase E6-associated protein (E6AP) and target the tumor suppressor p53 for ubiquitin-mediated proteolysis. Currently, how this critical event is regulated is largely unknown. Here we report that activating transcription factor 3 (ATF3), a broad DNA damage sensor whose expression is frequently downregulated in cervical cancer, interacted with E6 and prevented p53 from ubiquitination and degradation mediated by the viral protein. Consistent with its role as a potent E6 antagonist, ATF3 expressed enforcedly in HPV-positive SiHa cells activated p53, leading to expression of p53-target genes (e.g. p21 and PUMA), cell cycle arrest and apoptotic cell death. The leucine zipper domain of ATF3 appears indispensable for these effects as an ATF3 mutant lacking this domain failed to interact with E6 and activate p53 in the cervical cancer cells. The prevention of p53 degradation was unlikely caused by binding of ATF3 to the tumor suppressor, but rather was a consequence of disruption of the E6-E6AP interaction by ATF3. These results indicate that ATF3 plays a key role in a mechanism defending against HPV-induced carcinogenesis, and could serve as a novel therapeutic target for HPV-positive cancers.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

