Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma patients receiving temozolomide
- PMID: 20167815
- PMCID: PMC2940590
- DOI: 10.1093/neuonc/nop034
Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma patients receiving temozolomide
Abstract
We assessed six-month progression-free survival (PFS) as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma multiforme (GBM) patients receiving temozolomide (TMZ). A total of 183 patients with newly diagnosed GBM enrolled in 3 phase II protocols at the University of California-San Francisco were included. Patients were treated with interventions based on the Stupp regimen, each with the added component of a second oral agent given concurrently with radiotherapy and TMZ, followed by its coadministration with adjuvant TMZ. We examined whether progression status at 2, 4, and 6 months predicted subsequent survival using the landmark analysis. The hazard ratios of death as a function of progression status were estimated based on the Cox proportional hazards model after adjustment for putative prognostic factors. Progression status at 2, 4, and 6 months were all consistently found to be strong predictors of subsequent survival in all studies. The study-specific hazard ratios associated with progression status at 6 months ranged from 2.03 to 3.39. The hazard ratios associated with the earlier time points (2- and 4-month progression) all exceeded 2 in magnitude, ranging from 2.29 to 4.73. P-values were statistically significant for all time points. In this report, we demonstrated a strong association between the endpoints of PFS at 2, 4, and 6 months and survival. Patients who showed the signs of early progression were at significantly higher risk of earlier death. Our analysis suggests that 6-month PFS may be an appropriate primary endpoint in the context of phase II upfront GBM trials in the TMZ era.
Figures
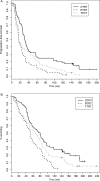
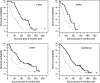
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med. 2005;352:987–996. - PubMed
-
- Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60:353–357. - PubMed
-
- Butowski N, Prados MD, Lamborn KR, et al. A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma. Int J Radiat Oncol Biol Phys. 2005;61:1454–1459. - PubMed
-
- Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

