Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma
- PMID: 20167817
- PMCID: PMC2940595
- DOI: 10.1093/neuonc/nop030
Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma
Abstract
The prognosis for patients with recurrent glioblastomas (GBMs) is dismal, with a median survival of 3-6 months. We performed a phase II trial of low-dose continuous (metronomic) treatment using temozolomide (TMZ) for recurrent GBMs. TMZ-refractory patients with GBM who experienced disease recurrence or progression during or after the cyclic treatment schedule of TMZ after surgery and standard radiotherapy were eligible. This phase II trial included 2 cohorts of patients. The initial cohort, comprising 10 patients, received TMZ at 40 mg/m(2) everyday. After this regimen seemed safe and effective, the metronomic schedule was changed to 50 mg/m(2) everyday. The second cohort, comprising 28 patients, received TMZ at 50 mg/m(2) everyday. The 6-month progression-free survival in all 38 patients was 32.5% (95% CI: 29.3%-35.8%) and the 6-month overall survival was 56.0% (95% CI: 36.2%-75.8%). One patient developed a grade III neutropenia, grade II thrombocytopenia in 3 patients, and grade II increase of liver enzyme (GOT/GPT) in 3 patients. Of all patients included in this study, 4 patients were withdrawn from this study because of side effects including sustained hematological disorders, cryptococcal infection, and cellulitis. In a response group, quality of life measured with short form-36 was well preserved, when compared with the pretreatment status. Metronomic treatment of TMZ is an effective treatment for recurrent GBM that is even refractory to conventional treatment of TMZ and has acceptable toxicity.
Figures
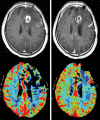
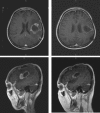
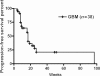
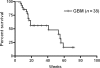
Similar articles
-
A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma.Oncol Rep. 2006 Nov;16(5):1117-21. Oncol Rep. 2006. PMID: 17016602 Clinical Trial.
-
Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study.J Clin Oncol. 2010 Apr 20;28(12):2051-7. doi: 10.1200/JCO.2009.26.5520. Epub 2010 Mar 22. J Clin Oncol. 2010. PMID: 20308655 Clinical Trial.
-
MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial.Clin Cancer Res. 2015 May 1;21(9):2057-64. doi: 10.1158/1078-0432.CCR-14-2737. Epub 2015 Feb 5. Clin Cancer Res. 2015. PMID: 25655102 Clinical Trial.
-
The efficacy of temozolomide for recurrent glioblastoma multiforme.Eur J Neurol. 2013 Feb;20(2):223-30. doi: 10.1111/j.1468-1331.2012.03778.x. Epub 2012 Jun 11. Eur J Neurol. 2013. PMID: 22680781 Review.
-
[Drug therapy of patients with recurrent glioblastoma: is there any evidence?].Wien Med Wochenschr. 2011 Jan;161(1-2):26-31. doi: 10.1007/s10354-010-0863-5. Epub 2010 Dec 27. Wien Med Wochenschr. 2011. PMID: 21181283 Review. German.
Cited by
-
Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas : A Prospective Multicenter Study of Korean Patients.J Korean Neurosurg Soc. 2012 Aug;52(2):92-7. doi: 10.3340/jkns.2012.52.2.92. Epub 2012 Aug 31. J Korean Neurosurg Soc. 2012. PMID: 23091665 Free PMC article.
-
Metronomic chemotherapy with daily low-dose temozolomide and celecoxib in elderly patients with newly diagnosed glioblastoma multiforme: a retrospective analysis.J Neurooncol. 2015 Sep;124(2):265-73. doi: 10.1007/s11060-015-1834-x. Epub 2015 Jun 5. J Neurooncol. 2015. PMID: 26045360
-
Efficacy and safety of metformin plus low-dose temozolomide in patients with recurrent or refractory glioblastoma: a randomized, prospective, multicenter, double-blind, controlled, phase 2 trial (KNOG-1501 study).Discov Oncol. 2023 Jun 6;14(1):90. doi: 10.1007/s12672-023-00678-3. Discov Oncol. 2023. PMID: 37278858 Free PMC article.
-
Incorporation of biomarkers in phase II studies of recurrent glioblastoma.Tumour Biol. 2015 Jan;36(1):153-62. doi: 10.1007/s13277-014-2960-3. Epub 2014 Dec 23. Tumour Biol. 2015. PMID: 25534238
-
A phase II study of feasibility and toxicity of bevacizumab in combination with temozolomide in patients with recurrent glioblastoma.Clin Transl Oncol. 2015 Sep;17(9):743-50. doi: 10.1007/s12094-015-1304-0. Epub 2015 Jun 2. Clin Transl Oncol. 2015. PMID: 26033428 Clinical Trial.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Brada M, Hoang-Xuan K, Rampling R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. - PubMed
-
- Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. - PubMed
-
- Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–740. - PubMed
-
- Jaeckle KA, Hess KR, Yung WK, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium Study. J Clin Oncol. 2003;21:2305–2311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

