Pharmacological targeting of the mitochondrial phosphatase PTPMT1
- PMID: 20167843
- PMCID: PMC2872949
- DOI: 10.1124/jpet.109.163329
Pharmacological targeting of the mitochondrial phosphatase PTPMT1
Abstract
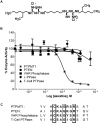
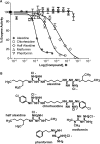
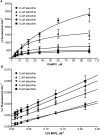
The dual-specificity protein tyrosine phosphatases (PTPs) play integral roles in the regulation of cell signaling. There is a need for new tools to study these phosphatases, and the identification of inhibitors potentially affords not only new means for their study, but also possible therapeutics for the treatment of diseases caused by their dysregulation. However, the identification of selective inhibitors of the protein phosphatases has proven somewhat difficult. PTP localized to mitochondrion 1 (PTPMT1) is a recently discovered dual-specificity phosphatase that has been implicated in the regulation of insulin secretion. Screening of a commercially available small-molecule library yielded alexidine dihydrochloride, a dibiguanide compound, as an effective and selective inhibitor of PTPMT1 with an in vitro concentration that inhibits response by 50% of 1.08 microM. A related dibiguanide analog, chlorhexidine dihydrochloride, also significantly inhibited PTPMT1, albeit with lower potency, while a monobiguanide analog showed very weak inhibition. Treatment of isolated rat pancreatic islets with alexidine dihydrochloride resulted in a dose-dependent increase in insulin secretion, whereas treatment of a pancreatic beta-cell line with the drug affected the phosphorylation of mitochondrial proteins in a manner similar to genetic inhibition of PTPMT1. Furthermore, knockdown of PTPMT1 in rat islets rendered them insensitive to alexidine dihydrochloride treatment, providing evidence for mechanism-based activity of the inhibitor. Taken together, these studies establish alexidine dihydrochloride as an effective inhibitor of PTPMT1, both in vitro and in cells, and support the notion that PTPMT1 could serve as a pharmacological target in the treatment of type II diabetes.
Figures
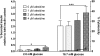

Similar articles
-
Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells.Mol Cell. 2005 Jul 22;19(2):197-207. doi: 10.1016/j.molcel.2005.06.008. Mol Cell. 2005. PMID: 16039589
-
Pharmacological targeting of the mitochondrial phosphatase PTPMT1 sensitizes hepatocellular carcinoma to ferroptosis.Cell Death Dis. 2025 Apr 6;16(1):257. doi: 10.1038/s41419-025-07581-5. Cell Death Dis. 2025. PMID: 40189563 Free PMC article.
-
PTPMT1 regulates mitochondrial death through the SLC25A6-NDUFS2 axis in pancreatic cancer cells.Am J Cancer Res. 2023 Mar 15;13(3):992-1003. eCollection 2023. Am J Cancer Res. 2023. PMID: 37034225 Free PMC article.
-
PTPMT1 inhibition induces apoptosis and growth arrest of human SCLC cells by disrupting mitochondrial metabolism.Transl Cancer Res. 2024 Dec 31;13(12):6956-6969. doi: 10.21037/tcr-2024-2379. Epub 2024 Dec 27. Transl Cancer Res. 2024. PMID: 39816544 Free PMC article.
-
Have we overlooked the importance of serine/threonine protein phosphatases in pancreatic beta-cells? Role played by protein phosphatase 2A in insulin secretion.JOP. 2005 Jul 8;6(4):303-15. JOP. 2005. PMID: 16006680 Review.
Cited by
-
Discovery of bactericides as an acute mitochondrial membrane damage inducer.Mol Biol Cell. 2021 Nov 1;32(21):ar32. doi: 10.1091/mbc.E21-04-0191. Epub 2021 Sep 8. Mol Biol Cell. 2021. PMID: 34495738 Free PMC article.
-
Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections.Microorganisms. 2020 Aug 26;8(9):1300. doi: 10.3390/microorganisms8091300. Microorganisms. 2020. PMID: 32858856 Free PMC article. Review.
-
The pseudophosphatase MK-STYX physically and genetically interacts with the mitochondrial phosphatase PTPMT1.PLoS One. 2014 Apr 7;9(4):e93896. doi: 10.1371/journal.pone.0093896. eCollection 2014. PLoS One. 2014. PMID: 24709986 Free PMC article.
-
Alexidine Dihydrochloride Has Broad-Spectrum Activities against Diverse Fungal Pathogens.mSphere. 2018 Oct 31;3(5):e00539-18. doi: 10.1128/mSphere.00539-18. mSphere. 2018. PMID: 30381356 Free PMC article.
-
Maintenance of mouse hematopoietic stem cells ex vivo by reprogramming cellular metabolism.Blood. 2015 Mar 5;125(10):1562-5. doi: 10.1182/blood-2014-04-568949. Epub 2015 Jan 15. Blood. 2015. PMID: 25593337 Free PMC article.
References
-
- Alessi DR, Smythe C, Keyse SM. (1993) The human CL100 gene encodes a Tyr/Thr-protein phosphatase, which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene 8:2015–2020 - PubMed
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. (2004) Protein tyrosine phosphatases in the human genome. Cell 117:699–711 - PubMed
-
- Charles CH, Abler AS, Lau LF. (1992) cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene 7:187–190 - PubMed
-
- Denu JM, Zhou G, Wu L, Zhao R, Yuvaniyama J, Saper MA, Dixon JE. (1995) The purification and characterization of a human dual-specific protein tyrosine phosphatase. J Biol Chem 270:3796–3803 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

