The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults
- PMID: 20167847
- PMCID: PMC2894413
- DOI: 10.1164/rccm.200910-1484OC
The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults
Abstract
Rationale: AERAS-402 is a novel tuberculosis vaccine designed to boost immunity primed by bacillus Calmette-Guérin (BCG), the only licensed vaccine.
Objectives: We investigated the safety and immunogenicity of AERAS-402 in healthy Mycobacterium tuberculosis-uninfected BCG-vaccinated adults from a tuberculosis-endemic region of South Africa.
Methods: Escalating doses of AERAS-402 vaccine were administered intramuscularly to each of three groups of healthy South African BCG-vaccinated adults, and a fourth group received two injections of the maximal dose. Participants were monitored for 6 months, with all adverse effects documented. Vaccine-induced CD4(+) and CD8(+) T-cell immunity was characterized by an intracellular cytokine staining assay of whole blood and peripheral blood mononuclear cells.
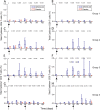
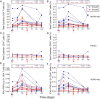
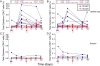
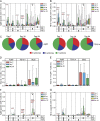
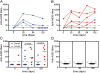
Measurements and main results: AERAS-402 was well tolerated, and no vaccine-related serious adverse events were recorded. The vaccine induced a robust CD4(+) T-cell response dominated by cells coexpressing IFN-gamma, tumor necrosis factor-alpha, and IL-2 ("polyfunctional" cells). AERAS-402 also induced a potent CD8(+) T-cell response, characterized by cells expressing IFN-gamma and/or tumor necrosis factor-alpha, which persisted for the duration of the study.
Conclusions: Vaccination with AERAS-402 is safe and immunogenic in healthy adults. The immunity induced by the vaccine appears promising: polyfunctional T cells are thought to be important for protection against intracellular pathogens such as Mycobacterium tuberculosis, and evidence is accumulating that CD8(+) T cells are also important. AERAS-402 induced a robust and durable CD8(+) T-cell response, which appears extremely promising. Clinical trial registered with www.sanctr.gov.za (NHREC no. 1381).
Figures
References
-
- World Health Organization. World Health Organization report: global tuberculosis control—epidemiology, strategy, financing. WHO/htm/TB/2009.411. Geneva, Switzerland: World Health Organization; 2009.
-
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993;22:1154–1158. - PubMed
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006;367:1173–1180. - PubMed
-
- Fine PA, Milstein J, Clements C. Issues relating to the use of BCG in immunization programmes: a discussion document. 1999. WHO/V&B/99.23. Available from: http://www.who.int/vaccines-documents/DocsPDF99www9943.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

