Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis
- PMID: 20167854
- PMCID: PMC2894410
- DOI: 10.1164/rccm.200906-0978OC
Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis
Abstract
Rationale: Inhaled granulocyte/macrophage-colony stimulating factor (GM-CSF) is a promising therapy for pulmonary alveolar proteinosis (PAP) but has not been adequately studied.
Objectives: To evaluate safety and efficacy of inhaled GM-CSF in patients with unremitting or progressive PAP.
Methods: We conducted a national, multicenter, self-controlled, phase II trial at nine pulmonary centers throughout Japan. Patients who had lung biopsy or cytology findings diagnostic of PAP, an elevated serum GM-CSF antibody level, and a Pa(O(2)) of less than 75 mm Hg entered a 12-week observation period. Those who improved (i.e., alveolar-arterial oxygen difference [A-aDO(2)] decreased by 10 mm Hg) during observation were excluded. The rest entered sequential periods of high-dose therapy (250 microg Days 1-8, none Days 9-14; x six cycles; 12 wk); low-dose therapy (125 microg Days 1-4, none Days 5-14; x six cycles; 12 wk), and follow-up (52 wk).
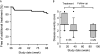
Measurements and main results: Fifty patients with PAP were enrolled in the study. During observation, nine improved and two withdrew; all of these were excluded. Of 35 patients completing the high- and low-dose therapy, 24 improved, resulting in an overall response rate of 62% (24/39; intention-to-treat analysis) and reduction in A-aDO(2) of 12.3 mm Hg (95% confidence interval, 8.4-16.2; n = 35, P < 0.001). No serious adverse events occurred, and serum GM-CSF autoantibody levels were unchanged. A treatment-emergent correlation occurred between A-aDO(2) and diffusing capacity of the lung, and high-resolution CT revealed improvement of ground-glass opacity. Twenty-nine of 35 patients remained stable without further therapy for 1 year.
Conclusions: Inhaled GM-CSF therapy is safe, effective, and provides a sustained therapeutic effect in autoimmune PAP. Clinical trial registered with www.controlled-trials.com/isrctn (ISRCTN18931678), www.jmacct.med.or.jp/english (JMA-IIA00013).
Figures




References
-
- Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med 1958;258:1123–1142. - PubMed
-
- Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 2004;103:1089–1098. - PubMed
-
- Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527–2539. - PubMed
-
- Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, Jobe AH. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol 1996;270:L650–L658. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

