Adriamycin nephropathy: a failure of endothelial progenitor cell-induced repair
- PMID: 20167859
- PMCID: PMC2843460
- DOI: 10.2353/ajpath.2010.091071
Adriamycin nephropathy: a failure of endothelial progenitor cell-induced repair
Abstract
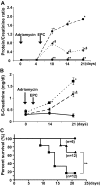
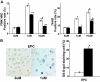
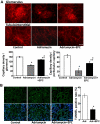
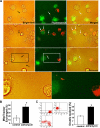
Adriamycin-associated nephropathy (AAN) remains poorly understood. We hypothesized that adriamycin affects endothelial progenitor cells (EPCs), leading to impaired regeneration. We analyzed renal hematopoietic stem cells (HSCs) and EPCs in mice with AAN and examined the potential contribution of adoptive transfer of intact EPCs to the repair processes. FACS analyses revealed that populations of HSCs and EPCs were scarcely represented in control kidneys and did not change numerically in kidneys obtained from mice with AAN. The observed defect in engraftment was attributable to the decreased viability and increased senescence of EPCs. Adoptive transfer of intact EPCs improved proteinuria and renal function, with a threefold decrease in mortality. Infusion of EPCs to adriamycin-treated mice reduced plasma levels of interleukin-1alpha and -beta and granulocyte-colony stimulating factor as well as increased the level of vascular endothelial growth factor with concomitant improvement of vascular density and reduction of apoptosis. An additional mechanism of tissue repair is proposed based on tunneling nanotube formation between EPCs and endothelial cells exposed to adriamycin, leading to the multiple rounds of exchange between EPCs and mature cells. In conclusion, AAN is associated with development of EPC incompetence; adoptive transfer of intact EPCs blunts morphological and functional manifestations of AAN; and the proposed mechanisms of repair by EPCs include direct incorporation into blood vessels, paracrine signaling, and tunneling nanotube renewal of mitochondrial pool in endothelial cells.
Figures

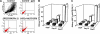


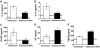
Comment in
-
First heal thyself: rescue of dysfunctional endothelial progenitor cells restores function to the injured kidney.Am J Pathol. 2010 Apr;176(4):1586-7. doi: 10.2353/ajpath.2010.091282. Epub 2010 Feb 25. Am J Pathol. 2010. PMID: 20185577 Free PMC article.
References
-
- Burke J, Laucius J, Brodovsky H, Soriano R. Doxorubicin hydrochloride-associated renal failure. Arch Intern Med. 1977;137:385–388. - PubMed
-
- Guest I, Uetrecht J. Drugs toxic to the bone marrow that target the stromal cells. Immunopharmacology. 2000;46:103–112. - PubMed
-
- Couture L, Nash J, Turgeon J. The ATP-binding cassette transporters and their implication in drug disposition: a special look at the heart. Pharmacol. 2006;Rev 58:244–258. - PubMed
-
- Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook T. Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J Immunol. 2006;177:4094–4102. - PubMed
-
- Kramer A, Hoven van den M, Rops A, Wijnhoven T, Heuvel van den L, Lensen J, van Kuppevelt T, van Goor H, van den Vlag J, Navis G, Berden J. Induction of glomerular heparanase expression in rats with adriamycin nephropathy is regulated by reactive oxygen species and the rennin-angiotensin system. J Am Soc Nephrol. 2006;17:2513–2520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

