Toll-like receptor 3 ligand dampens liver inflammation by stimulating Valpha 14 invariant natural killer T cells to negatively regulate gammadeltaT cells
- PMID: 20167870
- PMCID: PMC2843469
- DOI: 10.2353/ajpath.2010.090738
Toll-like receptor 3 ligand dampens liver inflammation by stimulating Valpha 14 invariant natural killer T cells to negatively regulate gammadeltaT cells
Abstract
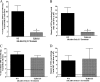
Valpha14 invariant natural killer T (Valpha14iNKT) cells are at the interface between the innate and adaptive immune responses and are thus critical for providing full engagement of host defense. We investigated the role of polyriboinosinic:polycytidylic acid (poly I:C), a replication-competent viral double-stranded RNA mimic and a specific agonist that recognizes the cellular sensor Toll-like receptor 3 (TLR3), in regulating Valpha14iNKT cell activation. We established for the first time that hepatic Valpha14iNKT cells up-regulate TLR3 extracellularly after poly I:C treatment. Notably, activation of TLR3-expressing hepatic Valpha14iNKT cells by a TLR3 ligand was suppressed by TLR3 deficiency. Our studies also revealed that Valpha14iNKT cell activation in response to poly I:C administration uniquely suppressed the accumulation and activation of intrahepatic gammadeltaT cells (but not natural killer cells) by inducing apoptosis. Furthermore, we established that activated hepatic Valpha14iNKT cells (via cytokines and possibly reactive oxygen species) influenced the frequency and absolute number of intrahepatic gammadeltaT cells, as evidenced by increased hepatic gammadeltaT cell accumulation in Valpha14iNKT cell-deficient mice after poly I:C treatment relative to wild-type mice. Thus, hepatic Valpha14iNKT cells and intrahepatic gammadeltaT cells are functionally linked on application of TLR3 agonist. Overall, our results demonstrate a novel and previously unrecognized anti-inflammatory role for activated hepatic Valpha14iNKT cells in negatively regulating intrahepatic gammadeltaT cell accumulation (probably through TLR3 signaling) and thereby preventing potentially harmful activation of intrahepatic gammadeltaT cells.
Figures


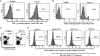
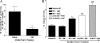


Similar articles
-
The ROS scavenger, NAC, regulates hepatic Vα14iNKT cells signaling during Fas mAb-dependent fulminant liver failure.PLoS One. 2012;7(6):e38051. doi: 10.1371/journal.pone.0038051. Epub 2012 Jun 6. PLoS One. 2012. PMID: 22701598 Free PMC article.
-
Invariant natural killer T cell deficiency leads to the development of spontaneous liver inflammation dependent on γδT cells in mice.J Gastroenterol. 2015 Nov;50(11):1124-33. doi: 10.1007/s00535-015-1060-5. Epub 2015 Mar 21. J Gastroenterol. 2015. PMID: 25791517
-
Interleukin 17-producing γδT cells promote hepatic regeneration in mice.Gastroenterology. 2014 Aug;147(2):473-84.e2. doi: 10.1053/j.gastro.2014.04.042. Epub 2014 May 4. Gastroenterology. 2014. PMID: 24801349 Free PMC article.
-
TLR3: interferon induction by double-stranded RNA including poly(I:C).Adv Drug Deliv Rev. 2008 Apr 29;60(7):805-12. doi: 10.1016/j.addr.2007.11.005. Epub 2008 Jan 2. Adv Drug Deliv Rev. 2008. PMID: 18262679 Review.
-
Targeting Cytokine Signals to Enhance γδT Cell-Based Cancer Immunotherapy.Front Immunol. 2022 Jun 7;13:914839. doi: 10.3389/fimmu.2022.914839. eCollection 2022. Front Immunol. 2022. PMID: 35747139 Free PMC article. Review.
Cited by
-
TLRs in Hepatic Cellular Crosstalk.Gastroenterol Res Pract. 2010;2010:618260. doi: 10.1155/2010/618260. Epub 2010 Aug 30. Gastroenterol Res Pract. 2010. PMID: 20862346 Free PMC article.
-
Cytokine dependent and independent iNKT cell activation.Cytokine. 2010 Sep;51(3):227-31. doi: 10.1016/j.cyto.2010.04.016. Cytokine. 2010. PMID: 20554220 Free PMC article. Review.
-
Toll-like receptor 3 in liver diseases.Gastroenterol Res Pract. 2010;2010:750904. doi: 10.1155/2010/750904. Epub 2010 Sep 23. Gastroenterol Res Pract. 2010. PMID: 20936107 Free PMC article.
-
Recent advances in different interactions between toll-like receptors and hepatitis B infection: a review.Front Immunol. 2024 Mar 13;15:1363996. doi: 10.3389/fimmu.2024.1363996. eCollection 2024. Front Immunol. 2024. PMID: 38545106 Free PMC article. Review.
-
The ROS scavenger, NAC, regulates hepatic Vα14iNKT cells signaling during Fas mAb-dependent fulminant liver failure.PLoS One. 2012;7(6):e38051. doi: 10.1371/journal.pone.0038051. Epub 2012 Jun 6. PLoS One. 2012. PMID: 22701598 Free PMC article.
References
-
- Van Kaer L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. - PubMed
-
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. - PubMed
-
- Wilson MT, Singh AK, Van Kaer L. Immunotherapy with ligands of natural killer T cells. Trends Mol Med. 2002;8:225–231. - PubMed
-
- Wilson MT, Van Kaer L. Natural killer T cells as targets for therapeutic intervention in autoimmune diseases. Curr Pharm Des. 2003;9:201–220. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

