Cyclic AMP- and (Rp)-cAMPS-induced conformational changes in a complex of the catalytic and regulatory (RI{alpha}) subunits of cyclic AMP-dependent protein kinase
- PMID: 20167947
- PMCID: PMC2957725
- DOI: 10.1074/mcp.M900388-MCP200
Cyclic AMP- and (Rp)-cAMPS-induced conformational changes in a complex of the catalytic and regulatory (RI{alpha}) subunits of cyclic AMP-dependent protein kinase
Abstract
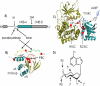
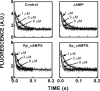
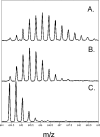
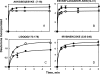
We took a discovery approach to explore the actions of cAMP and two of its analogs, one a cAMP mimic ((S(p))-adenosine cyclic 3':5'-monophosphorothioate ((S(p))-cAMPS)) and the other a diastereoisomeric antagonist ((R(p))-cAMPS), on a model system of the type Iα cyclic AMP-dependent protein kinase holoenzyme, RIα(91-244)·C-subunit, by using fluorescence spectroscopy and amide H/(2)H exchange mass spectrometry. Specifically, for the fluorescence experiments, fluorescein maleimide was conjugated to three cysteine single residue substitution mutants, R92C, T104C, and R239C, of RIα(91-244), and the effects of cAMP, (S(p))-cAMPS, and (R(p))-cAMPS on the kinetics of R-C binding and the time-resolved anisotropy of the reporter group at each conjugation site were measured. For the amide exchange experiments, ESI-TOF mass spectrometry with pepsin proteolytic fragmentation was used to assess the effects of (R(p))-cAMPS on amide exchange of the RIα(91-244)·C-subunit complex. We found that cAMP and its mimic perturbed at least parts of the C-subunit interaction Sites 2 and 3 but probably not Site 1 via reduced interactions of the linker region and αC of RIα(91-244). Surprisingly, (R(p))-cAMPS not only increased the affinity of RIα(91-244) toward the C-subunit by 5-fold but also produced long range effects that propagated through both the C- and R-subunits to produce limited unfolding and/or enhanced conformational flexibility. This combination of effects is consistent with (R(p))-cAMPS acting by enhancing the internal entropy of the R·C complex. Finally, the (R(p))-cAMPS-induced increase in affinity of RIα(91-244) toward the C-subunit indicates that (R(p))-cAMPS is better described as an inverse agonist because it decreases the fractional dissociation of the cyclic AMP-dependent protein kinase holoenzyme and in turn its basal activity.
Figures



Similar articles
-
Crystal structures of RIalpha subunit of cyclic adenosine 5'-monophosphate (cAMP)-dependent protein kinase complexed with (Rp)-adenosine 3',5'-cyclic monophosphothioate and (Sp)-adenosine 3',5'-cyclic monophosphothioate, the phosphothioate analogues of cAMP.Biochemistry. 2004 Jun 1;43(21):6620-9. doi: 10.1021/bi0302503. Biochemistry. 2004. PMID: 15157095
-
Cyclic-AMP and pseudosubstrate effects on type-I A-kinase regulatory and catalytic subunit binding kinetics.Biochemistry. 2007 Aug 14;46(32):9283-91. doi: 10.1021/bi700421h. Epub 2007 Jul 21. Biochemistry. 2007. PMID: 17658893
-
Mapping intersubunit interactions of the regulatory subunit (RIalpha) in the type I holoenzyme of protein kinase A by amide hydrogen/deuterium exchange mass spectrometry (DXMS).J Mol Biol. 2004 Jul 23;340(5):1185-96. doi: 10.1016/j.jmb.2004.05.042. J Mol Biol. 2004. PMID: 15236976
-
Chemoprevention with protein kinase A RIalpha antisense in DMBA-mammary carcinogenesis.Ann N Y Acad Sci. 2005 Nov;1058:255-64. doi: 10.1196/annals.1359.038. Ann N Y Acad Sci. 2005. PMID: 16394142 Review.
-
Dynamics of signaling by PKA.Biochim Biophys Acta. 2005 Dec 30;1754(1-2):25-37. doi: 10.1016/j.bbapap.2005.08.024. Epub 2005 Sep 22. Biochim Biophys Acta. 2005. PMID: 16214430 Review.
Cited by
-
Allosteric pluripotency as revealed by protein kinase A.Sci Adv. 2020 Jun 19;6(25):eabb1250. doi: 10.1126/sciadv.abb1250. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32596469 Free PMC article.
-
Phosphodiesterases catalyze hydrolysis of cAMP-bound to regulatory subunit of protein kinase A and mediate signal termination.Mol Cell Proteomics. 2011 Feb;10(2):M110.002295. doi: 10.1074/mcp.M110.002295. Epub 2010 Oct 5. Mol Cell Proteomics. 2011. PMID: 20923972 Free PMC article.
-
Allosteric inhibition explained through conformational ensembles sampling distinct "mixed" states.Comput Struct Biotechnol J. 2020 Nov 11;18:3803-3818. doi: 10.1016/j.csbj.2020.10.026. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33335680 Free PMC article. Review.
-
PDE-Mediated Cyclic Nucleotide Compartmentation in Vascular Smooth Muscle Cells: From Basic to a Clinical Perspective.J Cardiovasc Dev Dis. 2021 Dec 22;9(1):4. doi: 10.3390/jcdd9010004. J Cardiovasc Dev Dis. 2021. PMID: 35050214 Free PMC article. Review.
-
Signaling through dynamic linkers as revealed by PKA.Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14231-6. doi: 10.1073/pnas.1312644110. Epub 2013 Aug 14. Proc Natl Acad Sci U S A. 2013. PMID: 23946424 Free PMC article.
References
-
- Beebe S. J., Corbin J. D. (1986) Cyclic nucleotide-dependent protein kinases. Enzymes 17, 43–111
-
- Johnson D. A., Akamine P., Radzio-Andzelm E., Madhusudan M., Taylor S. S. (2001) Dynamics of cAMP-dependent protein kinase. Chem. Rev. 101, 2243–2270 - PubMed
-
- Shabb J. B. (2001) Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 101, 2381–2411 - PubMed
-
- Walsh D. A., Perkins J. P., Krebs E. G. (1968) An adenosine 3′,5′-monophosphate-dependent protein kinase from rabbit skeletal muscle. J. Biol. Chem. 243, 3763–3765 - PubMed
-
- Wu J., Brown S., Xuong N. H., Taylor S. S. (2004) RIalpha subunit of PKA: a cAMP-free structure reveals a hydrophobic capping mechanism for docking cAMP into site B. Structure 12, 1057–1065 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

