Factors associated with HIV RNA levels in pregnant women on non-suppressive highly active antiretroviral therapy at conception
- PMID: 20167990
- PMCID: PMC3428879
- DOI: 10.3851/IMP1489
Factors associated with HIV RNA levels in pregnant women on non-suppressive highly active antiretroviral therapy at conception
Abstract
Background: Little is known about pregnancy patterns and levels of HIV RNA in HIV-infected women conceiving on highly active antiretroviral therapy (HAART) with non-suppressed viral load (VL), nor about their therapeutic management.
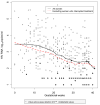
Methods: Linear mixed models were fitted to study changes in VL and potential associated factors including HAART type or duration and immune status among 127 women receiving HAART at conception with detectable VL enrolled in the prospective European Collaborative Study.
Results: Median duration of HAART at conception was 10 months. A total of 78 (61%) women conceived while on protease inhibitor (PI)-based HAART. Overall, 72 (57%) women remained on the same HAART regimen throughout pregnancy, 24 (19%) switched regimens and 31 (24%) interrupted HAART during early pregnancy. The intention-to-treat model indicated constant VL up to 10 gestational weeks; thereafter, levels decreased significantly, by 0.06 log(10) copies/ml weekly until delivery. At baseline, immune status was significantly associated with HIV RNA levels. Excluding those with treatment interruption, there was no significant difference in VL slope between women who did and did not modify their HAART regimens (P=0.14); women conceiving on non-nucleoside reverse transcriptase inhibitor-based HAART had consistently lower VL throughout pregnancy than those on PI-based HAART (P=0.02). Most (64/103, 62%) women had detectable VL within 4 weeks of delivery (median 2.40 log(10) copies/ml). The overall mother-to-child transmission rate was 1.72% (95% confidence interval 0.21-6.1).
Conclusions: Practices regarding management of women conceiving on HAART with detectable VL vary in Western Europe. The existence of this group of pregnant women highlights the need for improved monitoring of and support for treated women before they become pregnant, as well as during pregnancy.
Figures
References
-
- Thorne C, Newell ML. Antenatal and neonatal antiretroviral therapy in HIV-infected women and their infants: a review of safety issues. Med Wieku Rozwoj. 2003;7:425–36. - PubMed
-
- Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. - PubMed
-
- Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–84. - PubMed
-
- European Collaborative Study Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–65. - PubMed
-
- Jasseron C, Mandelbrot L, Tubiana R, et al. Prevention of mother-to-child HIV transmission: similar access for sub-Sahara African immigrants and for French women? AIDS. 2008;22:1503–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous