Pharmacokinetics of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian children
- PMID: 20167994
- PMCID: PMC2867092
- DOI: 10.3851/IMP1488
Pharmacokinetics of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian children
Abstract
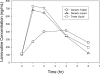
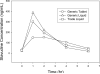
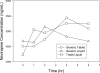
Background: The aim of this study was to evaluate the pharmacokinetics of lamivudine (3TC), stavudine (d4T) and nevirapine (NVP) in HIV-infected Malawian children receiving quartered tablet multiples of Triomune 40 (generic tablet [GT]) compared with individual generic liquid (GL) and trade liquid (TL).
Methods: This was a prospective randomized three-way crossover study. Patients (8-<12 kg, 18-<22 kg or 28-<32 kg body weight) taking Triomune 40 were recruited and randomized to receive GT twice daily (one-quarter, one-half or three-quarter tablets using Malawi treatment guidelines), GL twice daily (in the equivalent dose of GT) or TL twice daily (dosed using weight and age from US Department of Health and Human Services paediatric treatment guidelines). After 10 days of one formulation, 6-h pharmacokinetic sampling was performed, and patients were crossed over to subsequent formulations. Baseline concentration (C(0 h)), area under the curve (AUC)(0-6 h), maximum plasma concentration (C(max)) and time to C(max) were generated for each antiretroviral treatment.
Results: A total of 7 males and 11 females (6 in each GT dosing group) with a median (range) age of 7.2 years (1.3-13.6), weight of 19 kg (9.0-30.5) and height of 109 cm (75-132) were recruited. Combining all patients, no difference in pharmacokinetics was noted among the formulations for all drugs. However, patients in the one-quarter GT dosing group (8-<12 kg) had lower 3TC exposures than with the GL or TL (3TC AUC(0-6 h) 1,102, 1,720 and 2,060 h*ng/ml, respectively; P<0.005) and had more subtherapeutic NVP C(0 h) (10 of 13 occasions versus the one-half and three-quarter tablet groups). Compared with Western paediatric cohorts, Malawians had concentrations 30-40% lower for 3TC and d4T and 50% higher for NVP.
Conclusions: Quartered multiples of Triomune 40 are appropriate for children 18-<22 kg and 28-<32 kg in weight; however, alternative formulations are suggested in children weighing 8-<12 kg.
Figures
Similar articles
-
Pharmacokinetic comparison of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian adults.AIDS. 2007 Jan 2;21(1):59-64. doi: 10.1097/QAD.0b013e3280117ca0. AIDS. 2007. PMID: 17148968 Clinical Trial.
-
Steady state bioequivalence of generic and innovator formulations of stavudine, lamivudine, and nevirapine in HIV-infected Ugandan adults.PLoS One. 2008;3(12):e3981. doi: 10.1371/journal.pone.0003981. Epub 2008 Dec 19. PLoS One. 2008. PMID: 19096711 Free PMC article. Clinical Trial.
-
Steady-state nevirapine, lamivudine and stavudine levels in Malawian HIV-infected children on antiretroviral therapy using split Triomune 30 tablets.Antivir Ther. 2010;15(3):343-50. doi: 10.3851/IMP1544. Antivir Ther. 2010. PMID: 20516554 Free PMC article.
-
Steady-state pharmacokinetic comparison of generic and branded formulations of stavudine, lamivudine and nevirapine in HIV-infected Ugandan adults.J Antimicrob Chemother. 2008 Nov;62(5):1113-7. doi: 10.1093/jac/dkn290. Epub 2008 Jul 18. J Antimicrob Chemother. 2008. PMID: 18641036 Free PMC article. Clinical Trial.
-
Global challenges in the development and delivery of paediatric antiretrovirals.Drug Discov Today. 2008 Jun;13(11-12):530-5. doi: 10.1016/j.drudis.2008.03.018. Epub 2008 May 5. Drug Discov Today. 2008. PMID: 18549980 Free PMC article. Review.
Cited by
-
Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children.J Acquir Immune Defic Syndr. 2011 Dec 1;58(4):385-91. doi: 10.1097/QAI.0b013e318232b057. J Acquir Immune Defic Syndr. 2011. PMID: 21876444 Free PMC article. Clinical Trial.
-
The urgent need for newer drugs in routine HIV treatment in Africa: the case of Ghana.Front Epidemiol. 2025 Mar 14;5:1523109. doi: 10.3389/fepid.2025.1523109. eCollection 2025. Front Epidemiol. 2025. PMID: 40161547 Free PMC article. Review.
-
Antiretroviral drugs in pediatric HIV-infected patients: pharmacokinetic and practical challenges.Paediatr Drugs. 2011 Jun 1;13(3):175-92. doi: 10.2165/11587300-000000000-00000. Paediatr Drugs. 2011. PMID: 21500872 Review.
-
Sub-therapeutic nevirapine concentration during antiretroviral treatment initiation among children living with HIV: Implications for therapeutic drug monitoring.PLoS One. 2017 Aug 21;12(8):e0183080. doi: 10.1371/journal.pone.0183080. eCollection 2017. PLoS One. 2017. PMID: 28827836 Free PMC article.
-
Exploration of CYP450 and drug transporter genotypes and correlations with nevirapine exposure in Malawians.Pharmacogenomics. 2012 Jan;13(1):113-21. doi: 10.2217/pgs.11.132. Epub 2011 Nov 23. Pharmacogenomics. 2012. PMID: 22111602 Free PMC article.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS Epidemic Update 2007. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
-
- World Health Organization Malawi summary country profile for HIV/AIDS treatment scale-up 2005. www.who.int/hiv.
-
- Malawi Ministry of Health ART in the public and private sectors in Malawi: Results up to 30th December 2007.
-
- Penzak SR, Acosta EP, Tavel JA, Masur H. Analysis of generic nevirapine products in developing countries. JAMA. 2003;289:2648–49. - PubMed
-
- Narang VS, Lulla A, Malhotra G, Purandare S. A combined-formulation tablet of lamivudine/nevirapine/stavudine: bioequivalence compared with concurrent administration of lamivudine, nevirapine, and stavudine in healthy Indian subjects. J Clin Pharmacol. 2005;45:265–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

