Structural basis of transmembrane domain interactions in integrin signaling
- PMID: 20168080
- PMCID: PMC2900621
- DOI: 10.4161/cam.4.2.10592
Structural basis of transmembrane domain interactions in integrin signaling
Abstract
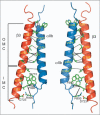
Cell surface receptors of the integrin family are pivotal to cell adhesion and migration. The activation state of heterodimeric alphabeta integrins is correlated to the association state of the single-pass alpha and beta transmembrane domains. The association of integrin alphaIIbbeta3 transmembrane domains, resulting in an inactive receptor, is characterized by the asymmetric arrangement of a straight (alphaIIb) and tilted (beta3) helix relative to the membrane in congruence to the dissociated structures. This allows for a continuous association interface centered on helix-helix glycine-packing and an unusual alphaIIb(GFF) structural motif that packs the conserved Phe-Phe residues against the beta3 transmembrane helix, enabling alphaIIb(D723)beta3(R995) electrostatic interactions. The transmembrane complex is further stabilized by the inactive ectodomain, thereby coupling its association state to the ectodomain conformation. In combination with recently determined structures of an inactive integrin ectodomain and an activating talin/beta complex that overlap with the alphabeta transmembrane complex, a comprehensive picture of integrin bi-directional transmembrane signaling has emerged.
Figures



Similar articles
-
Structure of the integrin alphaIIb transmembrane segment.J Biol Chem. 2008 Jun 6;283(23):16162-8. doi: 10.1074/jbc.M801748200. Epub 2008 Apr 16. J Biol Chem. 2008. PMID: 18417472 Free PMC article.
-
The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling.EMBO J. 2009 May 6;28(9):1351-61. doi: 10.1038/emboj.2009.63. Epub 2009 Mar 12. EMBO J. 2009. PMID: 19279667 Free PMC article.
-
Transmembrane interactions of alpha/beta integrin signaling.Structure. 2005 May;13(5):683-4. doi: 10.1016/j.str.2005.04.004. Structure. 2005. PMID: 15893656 No abstract available.
-
Structural basis of integrin transmembrane activation.J Cell Biochem. 2010 Feb 15;109(3):447-52. doi: 10.1002/jcb.22427. J Cell Biochem. 2010. PMID: 19950197 Review.
-
Linking integrin conformation to function.J Cell Sci. 2009 Jan 15;122(Pt 2):165-70. doi: 10.1242/jcs.018556. J Cell Sci. 2009. PMID: 19118208 Free PMC article. Review.
Cited by
-
Demonstration of novel gain-of-function mutations of αIIbβ3: association with macrothrombocytopenia and glanzmann thrombasthenia-like phenotype.Mol Genet Genomic Med. 2013 Jul;1(2):77-86. doi: 10.1002/mgg3.9. Epub 2013 Apr 22. Mol Genet Genomic Med. 2013. PMID: 24498605 Free PMC article.
-
Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors.Biol Open. 2012 Apr 15;1(4):329-40. doi: 10.1242/bio.2012364. Epub 2012 Feb 6. Biol Open. 2012. PMID: 23213423 Free PMC article.
-
Integrins as Therapeutic Targets: Successes and Cancers.Cancers (Basel). 2017 Aug 23;9(9):110. doi: 10.3390/cancers9090110. Cancers (Basel). 2017. PMID: 28832494 Free PMC article. Review.
-
The tail of integrin activation.Trends Biochem Sci. 2011 Apr;36(4):191-8. doi: 10.1016/j.tibs.2010.11.002. Epub 2011 Jan 6. Trends Biochem Sci. 2011. PMID: 21216149 Free PMC article. Review.
-
Inhibition of PlexA1-mediated brain tumor growth and tumor-associated angiogenesis using a transmembrane domain targeting peptide.Oncotarget. 2016 Sep 6;7(36):57851-57865. doi: 10.18632/oncotarget.11072. Oncotarget. 2016. PMID: 27506939 Free PMC article.
References
-
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. - PubMed
-
- Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J Thromb Haemost. 2009;7:200–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
