Receptor-mediated and fluid-phase transcytosis of horseradish peroxidase across rat hepatocytes
- PMID: 20168981
- PMCID: PMC2820271
- DOI: 10.1155/2010/850320
Receptor-mediated and fluid-phase transcytosis of horseradish peroxidase across rat hepatocytes
Abstract
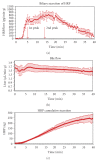
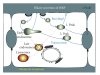
Horseradish peroxidase (HRP) is often used as a fluid-phase marker to characterize endocytic and transcytotic processes. Likewise, it has been applied to investigate the mechanisms of biliary secretion of fluid in rat liver hepatocytes. However, HRP contains mannose residues and thus binds to mannose receptors (MRs) on liver cells, including hepatocytes. To study the role of MR-mediated endocytosis of HRP transport in hepatocytes, we determined the influence of the oligosaccharid mannan on HRP biliary secretion in the isolated perfused rat liver. A 1-minute pulse of HRP was applied followed by marker-free perfusion. HRP appeared in bile with biphasic kinetics: a first peak at 7 minutes and a second peak at 15 minutes after labeling. Perfusion with 0.8 mg/mL HRP in the presence of a twofold excess of mannan reduced the first peak by 41% without effect on the second one. Together with recently published data on MR expression in rat hepatocytes this demonstrates two different mechanisms for HRP transcytosis: a rapid, receptor-mediated transport and a slower fluid-phase transport.
Figures



Similar articles
-
Biliary secretion of fluid phase markers is modified under post-cholestatic conditions.Wien Med Wochenschr. 2008;158(19-20):579-82. doi: 10.1007/s10354-008-0600-5. Wien Med Wochenschr. 2008. PMID: 18998077
-
Hepatocyte horseradish peroxidase uptake is saturable and inhibited by mannose-terminal glycoproteins.Am J Physiol. 1993 May;264(5 Pt 1):G880-5. doi: 10.1152/ajpgi.1993.264.5.G880. Am J Physiol. 1993. PMID: 8498515
-
Cell surface distribution and intracellular fate of asialoglycoproteins: a morphological and biochemical study of isolated rat hepatocytes and monolayer cultures.J Cell Biol. 1982 Mar;92(3):634-47. doi: 10.1083/jcb.92.3.634. J Cell Biol. 1982. PMID: 6282890 Free PMC article.
-
Effects of cyclosporin A on paracellular and transcellular transport of horseradish peroxidase in perfused rat livers.Dig Dis Sci. 1997 Mar;42(3):514-21. doi: 10.1023/a:1018834723417. Dig Dis Sci. 1997. PMID: 9073132
-
Targeting the mannose receptor with mannosylated subunit vaccines.Curr Med Chem. 2014;21(30):3405-18. doi: 10.2174/0929867321666140826115552. Curr Med Chem. 2014. PMID: 25174924 Review.
Cited by
-
Regulation of alternative macrophage activation in the liver following acetaminophen intoxication by stem cell-derived tyrosine kinase.Toxicol Appl Pharmacol. 2012 Jul 15;262(2):139-48. doi: 10.1016/j.taap.2012.04.027. Epub 2012 May 1. Toxicol Appl Pharmacol. 2012. PMID: 22575169 Free PMC article.
-
Technologies for investigating the physiological barriers to efficient lipid nanoparticle-siRNA delivery.J Histochem Cytochem. 2013 Jun;61(6):407-20. doi: 10.1369/0022155413484152. Epub 2013 Mar 14. J Histochem Cytochem. 2013. PMID: 23504369 Free PMC article. Review.
-
Advances in Understanding of Structural Reorganization in the Hypothalamic Neurosecretory System.Front Endocrinol (Lausanne). 2017 Oct 17;8:275. doi: 10.3389/fendo.2017.00275. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29089925 Free PMC article. Review.
-
Matrix-free human 2D organoids recapitulate duodenal barrier and transport properties.BMC Biol. 2025 Jan 5;23(1):2. doi: 10.1186/s12915-024-02105-7. BMC Biol. 2025. PMID: 39757172 Free PMC article.
-
Mannose receptor (MRC1) mediates uptake of dextran by bone marrow-derived macrophages.Mol Biol Cell. 2024 Dec 1;35(12):ar153. doi: 10.1091/mbc.E24-08-0355. Epub 2024 Nov 6. Mol Biol Cell. 2024. PMID: 39504444 Free PMC article.
References
-
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiological Reviews. 2003;83(3):871–932. - PubMed
-
- Dunn WA, Wall DA, Hubbard AL. Use of isolated, perfused liver in studies of receptor-mediated endocytosis. Methods in Enzymology. 1983;98:225–241. - PubMed
-
- Kloppel TM. Effects of temperature on the degradation and biliary secretion of asialoorosomucoid by the perfused rat liver: evidence for two intracellular pathways. Journal of Cellular Physiology. 1989;138(3):555–560. - PubMed
-
- Kloppel TM, Brown WR, Reichen J. Effects of monensin on vesicular transport pathways in the perfused rat liver. Journal of Cellular Biochemistry. 1986;32(3):235–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

