Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification
- PMID: 20168989
- PMCID: PMC2820409
- DOI: 10.1371/journal.ppat.1000790
Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification
Abstract
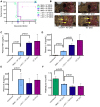
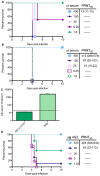
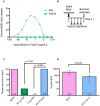
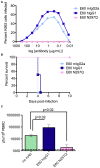
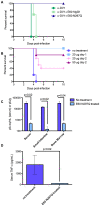
Immunity to one of the four dengue virus (DV) serotypes can increase disease severity in humans upon subsequent infection with another DV serotype. Serotype cross-reactive antibodies facilitate DV infection of myeloid cells in vitro by promoting virus entry via Fcgamma receptors (FcgammaR), a process known as antibody-dependent enhancement (ADE). However, despite decades of investigation, no in vivo model for antibody enhancement of dengue disease severity has been described. Analogous to human infants who receive anti-DV antibodies by transplacental transfer and develop severe dengue disease during primary infection, we show here that passive administration of anti-DV antibodies is sufficient to enhance DV infection and disease in mice using both mouse-adapted and clinical DV isolates. Antibody-enhanced lethal disease featured many of the hallmarks of severe dengue disease in humans, including thrombocytopenia, vascular leakage, elevated serum cytokine levels, and increased systemic viral burden in serum and tissue phagocytes. Passive transfer of a high dose of serotype-specific antibodies eliminated viremia, but lower doses of these antibodies or cross-reactive polyclonal or monoclonal antibodies all enhanced disease in vivo even when antibody levels were neutralizing in vitro. In contrast, a genetically engineered antibody variant (E60-N297Q) that cannot bind FcgammaR exhibited prophylactic and therapeutic efficacy against ADE-induced lethal challenge. These observations provide insight into the pathogenesis of antibody-enhanced dengue disease and identify a novel strategy for the design of therapeutic antibodies against dengue.
Conflict of interest statement
MSD has consulting agreements with MacroGenics, a company that has licensed the E60 monoclonal antibody from Washington University for possible commercial development. SJ is an employee of MacroGenics.
Figures


References
-
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. - PubMed
-
- WHO . Geneva: World Health Organization; 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention, and control.
-
- Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. - PubMed
-
- Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

