Interaction of botulinum toxin with the epithelial barrier
- PMID: 20169001
- PMCID: PMC2822237
- DOI: 10.1155/2010/974943
Interaction of botulinum toxin with the epithelial barrier
Abstract
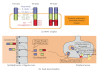
Botulinum neurotoxin (BoNT) is a protein toxin (approximately 150 kDa), which possesses a metalloprotease activity. Food-borne botulism is manifested when BoNT is absorbed from the digestive tract to the blood stream and enters the peripheral nerves, where the toxin cleaves core proteins of the neuroexocytosis apparatus and elicits the inhibition of neurotransmitter release. The initial obstacle to orally ingested BoNT entering the body is the epithelial barrier of the digestive tract. Recent cell biology and molecular biology studies are beginning to elucidate the mechanism by which this large protein toxin crosses the epithelial barrier. In this review, we provide an overview of the structural features of botulinum toxins (BoNT and BoNT complex) and the interaction of these toxins with the epithelial barrier.
Figures

References
-
- Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management. Journal of the American Medical Association. 2001;285(8):1059–1070. - PubMed
-
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiological Reviews. 2000;80(2):717–766. - PubMed
-
- Sakaguchi G. Clostridium botulinum toxins. Pharmacology & Therapeutics. 1982;19(2):165–194. - PubMed
-
- Minton NP. Molecular genetics of clostridial neurotoxins. Current Topics in Microbiology and Immunology. 1995;195:161–194. - PubMed
-
- Collins MD, East AK. Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. Journal of Applied Microbiology. 1998;84(1):5–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

