Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR
- PMID: 20169078
- PMCID: PMC2821926
- DOI: 10.1371/journal.pone.0009239
Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a commonly inherited renal disorder caused by defects in the PKD1 or PKD2 genes. ADPKD is associated with significant morbidity, and is a major underlying cause of end-stage renal failure (ESRF). Commonly, treatment options are limited to the management of hypertension, cardiovascular risk factors, dialysis, and transplantation when ESRF develops, although several new pharmacotherapies, including rapamycin, have shown early promise in animal and human studies. Evidence implicates polycystin-1 (PC-1), the gene product of the PKD1 gene, in regulation of the mTOR pathway. Here we demonstrate a mechanism by which the intracellular, carboxy-terminal tail of polycystin-1 (CP1) regulates mTOR signaling by altering the subcellular localization of the tuberous sclerosis complex 2 (TSC2) tumor suppressor, a gatekeeper for mTOR activity. Phosphorylation of TSC2 at S939 by AKT causes partitioning of TSC2 away from the membrane, its GAP target Rheb, and its activating partner TSC1 to the cytosol via 14-3-3 protein binding. We found that TSC2 and a C-terminal polycystin-1 peptide (CP1) directly interact and that a membrane-tethered CP1 protects TSC2 from AKT phosphorylation at S939, retaining TSC2 at the membrane to inhibit the mTOR pathway. CP1 decreased binding of 14-3-3 proteins to TSC2 and increased the interaction between TSC2 and its activating partner TSC1. Interestingly, while membrane tethering of CP1 was required to activate TSC2 and repress mTOR, the ability of CP1 to inhibit mTOR signaling did not require primary cilia and was independent of AMPK activation. These data identify a unique mechanism for modulation of TSC2 repression of mTOR signaling via membrane retention of this tumor suppressor, and identify PC-1 as a regulator of this downstream component of the PI3K signaling cascade.
Conflict of interest statement
Figures
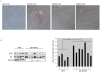
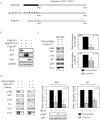


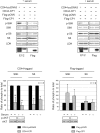


Similar articles
-
The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway.Hum Mol Genet. 2009 Jan 1;18(1):151-63. doi: 10.1093/hmg/ddn325. Epub 2008 Oct 9. Hum Mol Genet. 2009. PMID: 18845692 Free PMC article.
-
Insulin like growth factor-1-induced phosphorylation and altered distribution of tuberous sclerosis complex (TSC)1/TSC2 in C2C12 myotubes.FEBS J. 2010 May;277(9):2180-91. doi: 10.1111/j.1742-4658.2010.07635.x. FEBS J. 2010. PMID: 20412061 Free PMC article.
-
Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1.Mol Cell Biol. 2009 May;29(9):2359-71. doi: 10.1128/MCB.01259-08. Epub 2009 Mar 2. Mol Cell Biol. 2009. PMID: 19255143 Free PMC article.
-
A complex interplay between Akt, TSC2 and the two mTOR complexes.Biochem Soc Trans. 2009 Feb;37(Pt 1):217-22. doi: 10.1042/BST0370217. Biochem Soc Trans. 2009. PMID: 19143635 Free PMC article. Review.
-
Phosphorylation, protein kinases and ADPKD.Biochim Biophys Acta. 2011 Oct;1812(10):1219-24. doi: 10.1016/j.bbadis.2011.03.001. Epub 2011 Mar 15. Biochim Biophys Acta. 2011. PMID: 21392577 Review.
Cited by
-
Pediatric glioma-associated KIAA1549:BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner.Genes Dev. 2012 Dec 1;26(23):2561-6. doi: 10.1101/gad.200907.112. Epub 2012 Nov 14. Genes Dev. 2012. PMID: 23152448 Free PMC article.
-
Primary cilia and kidney injury: current research status and future perspectives.Am J Physiol Renal Physiol. 2013 Oct 15;305(8):F1085-98. doi: 10.1152/ajprenal.00399.2013. Epub 2013 Jul 31. Am J Physiol Renal Physiol. 2013. PMID: 23904226 Free PMC article. Review.
-
Polycystin-1 affects cancer cell behaviour and interacts with mTOR and Jak signalling pathways in cancer cell lines.J Cell Mol Med. 2019 Sep;23(9):6215-6227. doi: 10.1111/jcmm.14506. Epub 2019 Jun 28. J Cell Mol Med. 2019. PMID: 31251475 Free PMC article.
-
Structural insights into TSC complex assembly and GAP activity on Rheb.Nat Commun. 2021 Jan 12;12(1):339. doi: 10.1038/s41467-020-20522-4. Nat Commun. 2021. PMID: 33436626 Free PMC article.
-
14-3-3 binding motif phosphorylation disrupts Hdac4-organized condensates to stimulate cardiac reprogramming.Cell Rep. 2024 Apr 23;43(4):114054. doi: 10.1016/j.celrep.2024.114054. Epub 2024 Apr 4. Cell Rep. 2024. PMID: 38578832 Free PMC article.
References
-
- Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int J Biochem Cell Biol. 2004;36:1868–1873. - PubMed
-
- Chang MY, Ong AC. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and treatment. Nephron Physiol. 2008;108:1–7. - PubMed
-
- Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–2398. - PubMed
-
- Wilson PD, Goilav B. Cystic disease of the kidney. Annu Rev Pathol. 2007;2:341–368. - PubMed
-
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

