Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile
- PMID: 20169081
- PMCID: PMC2821929
- DOI: 10.1371/journal.pone.0009252
Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile
Abstract
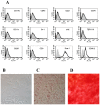
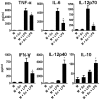
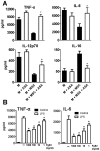
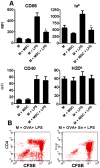
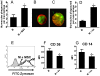
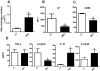
In recent years it has become clear that the therapeutic properties of bone marrow-derived mesenchymal stromal cells (MSC) are related not only to their ability to differentiate into different lineages but also to their capacity to suppress the immune response. We here studied the influence of MSC on macrophage function. Using mouse thioglycolate-elicited peritoneal macrophages (M) stimulated with LPS, we found that MSC markedly suppressed the production of the inflammatory cytokines TNF-alpha, IL-6, IL-12p70 and interferon-gamma while increased the production of IL-10 and IL-12p40. Similar results were observed using supernatants from MSC suggesting that factor(s) constitutively released by MSC are involved. Supporting a role for PGE(2) we observed that acetylsalicylic acid impaired the ability of MSC to inhibit the production of inflammatory cytokines and to stimulate the production of IL-10 by LPS-stimulated M. Moreover, we found that MSC constitutively produce PGE2 at levels able to inhibit the production of TNF-alpha and IL-6 by activated M. MSC also inhibited the up-regulation of CD86 and MHC class II in LPS-stimulated M impairing their ability to activate antigen-specific T CD4+ cells. On the other hand, they stimulated the uptake of apoptotic thymocytes by M. Of note, MSC turned M into cells highly susceptible to infection with the parasite Trypanosoma cruzi increasing more than 5-fold the rate of M infection. Using a model of inflammation triggered by s.c. implantation of glass cylinders, we found that MSC stimulated the recruitment of macrophages which showed a low expression of CD86 and the MHC class II molecule Ia(b) and a high ability to produce IL-10 and IL-12p40, but not IL-12 p70. In summary, our results suggest that MSC switch M into a regulatory profile characterized by a low ability to produce inflammatory cytokines, a high ability to phagocyte apoptotic cells, and a marked increase in their susceptibility to infection by intracellular pathogens.
Conflict of interest statement
Figures


References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Chamberlain G, Fox J, Ashton B, Middleton J. Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. - PubMed
-
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. - PubMed
-
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

