Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study
- PMID: 20169113
- PMCID: PMC2821896
- DOI: 10.1371/journal.pmed.1000233
Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study
Abstract
Background: Intrapartum and neonatal single-dose nevirapine (NVP) reduces the risk of mother-to-child HIV transmission but also induces viral resistance to non-nucleoside reverse transcriptase inhibitor (NNRTI) drugs. This drug resistance largely fades over time. We hypothesized that women with a prior single-dose NVP exposure would have no more than a 10% higher cumulative prevalence of failure of their NNRTI-containing antiretroviral therapy (ART) over the first 48 wk of therapy than would women without a prior exposure.
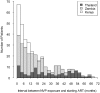
Methods and findings: We enrolled 355 NVP-exposed and 523 NVP-unexposed women at two sites in Zambia, one site in Kenya, and two sites in Thailand into a prospective, non-inferiority cohort study and followed them for 48 wk on ART. Those who died, discontinued NNRTI-containing ART, or had a plasma viral load >or=400 copies/ml at either the 24 wk or 48 wk study visits and confirmed on repeat testing were characterized as having failed therapy. Overall, 114 of 355 NVP-exposed women (32.1%) and 132 of 523 NVP-unexposed women (25.2%) met criteria for treatment failure. The difference in failure rates between the exposure groups was 6.9% (95% confidence interval [CI] 0.8%-13.0%). The failure rates of women stratified by our predefined exposure interval categories were as follows: 47 of 116 women in whom less than 6 mo elapsed between exposure and starting ART failed therapy (40%; p<0.001 compared to unexposed women); 25 of 67 women in whom 7-12 mo elapsed between exposure and starting ART failed therapy (37%; p = 0.04 compared to unexposed women); and 42 of 172 women in whom more than 12 mo elapsed between exposure and starting ART failed therapy (24%; p = 0.82 compared to unexposed women). Locally weighted regression analysis also indicated a clear inverse relationship between virologic failure and the exposure interval.
Conclusions: Prior exposure to single-dose NVP was associated with an increased risk of treatment failure; however, this risk seems largely confined to women with a more recent exposure. Women requiring ART within 12 mo of NVP exposure should not be prescribed an NNRTI-containing regimen as first-line therapy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization. The 3 by 5 initiative: AIDS in children. Available: http://www.who.int/3by5/paediatric/en/index.html. Accessed 7 February 2007.
-
- De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–1182. - PubMed
-
- Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. - PubMed
-
- Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. - PubMed
-
- Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

