Detection of Wuchereria bancrofti L3 larvae in mosquitoes: a reverse transcriptase PCR assay evaluating infection and infectivity
- PMID: 20169115
- PMCID: PMC2821903
- DOI: 10.1371/journal.pntd.0000602
Detection of Wuchereria bancrofti L3 larvae in mosquitoes: a reverse transcriptase PCR assay evaluating infection and infectivity
Abstract
Background: Detection of filarial DNA in mosquitoes by PCR cannot differentiate infective mosquitoes from infected mosquitoes. In order to evaluate transmission risk an assay is needed that can specifically detect infective L3 stage parasites. We now report the development of an assay that specifically detects the infective stage of Wuchereria bancrofti in mosquitoes. The assay detects an L3-activated mRNA transcript by reverse-transcriptase PCR (RT-PCR).
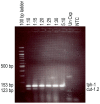
Methodology/principal findings: W. bancrofti cuticle-related genes were selected using bioinformatics and screened as potential diagnostic target genes for L3 detection in mosquitoes. Expression profiles were determined using RT-PCR on RNA isolated from mosquitoes collected daily across a two-week period after feeding on infected blood. Conventional multiplex RT-PCR and real-time multiplex RT-PCR assays were developed using an L3-activated cuticlin transcript for L3 detection and a constitutively expressed transcript, tph-1, for 'any-stage' detection.
Conclusions/significance: This assay can be used to simultaneously detect W. bancrofti infective stage larvae and 'any-stage' larvae in pooled vector mosquitoes. This test may be useful as a tool for assessing changes in transmission potential in the context of filariasis elimination programs.
Conflict of interest statement
GJW is on the Editorial Board of
Figures

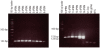
Similar articles
-
RT-PCR assay for the detection of infective (L3) larvae of lymphatic filarial parasite, Wuchereria bancrofti, in vector mosquito Culex quinquefasciatus.J Vector Borne Dis. 2008 Sep;45(3):207-16. J Vector Borne Dis. 2008. PMID: 18807377
-
A reverse transcriptase-PCR assay for detecting filarial infective larvae in mosquitoes.PLoS Negl Trop Dis. 2008 Jun 18;2(6):e251. doi: 10.1371/journal.pntd.0000251. PLoS Negl Trop Dis. 2008. PMID: 18560545 Free PMC article.
-
Multi-centric evaluation of a stage-specific reverse transcriptase-polymerase chain reaction assay as a xenomonitoring tool for the detection of infective (L3) stage Wuchereria bancrofti in vectors.Indian J Med Res. 2021 Jul;154(1):132-140. doi: 10.4103/ijmr.IJMR_713_19. Indian J Med Res. 2021. PMID: 34782539 Free PMC article.
-
Application of biotechnology in the identification of filarial larva in mosquitoes.Southeast Asian J Trop Med Public Health. 1988 Mar;19(1):87-9. Southeast Asian J Trop Med Public Health. 1988. PMID: 2900557 Review.
-
DNA-based diagnosis of lymphatic filariasis.Southeast Asian J Trop Med Public Health. 2009 Sep;40(5):904-13. Southeast Asian J Trop Med Public Health. 2009. PMID: 19842372 Review.
Cited by
-
Assessing the Presence of Wuchereria bancrofti Infections in Vectors Using Xenomonitoring in Lymphatic Filariasis Endemic Districts in Ghana.Trop Med Infect Dis. 2019 Mar 17;4(1):49. doi: 10.3390/tropicalmed4010049. Trop Med Infect Dis. 2019. PMID: 30884886 Free PMC article.
-
Assessing the presence of Wuchereria bancrofti in vector and human populations from urban communities in Conakry, Guinea.Parasit Vectors. 2015 Sep 26;8:492. doi: 10.1186/s13071-015-1077-x. Parasit Vectors. 2015. PMID: 26410739 Free PMC article.
-
Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia.PLoS Negl Trop Dis. 2011 Aug;5(8):e1299. doi: 10.1371/journal.pntd.0001299. Epub 2011 Aug 30. PLoS Negl Trop Dis. 2011. PMID: 21912716 Free PMC article.
-
Lymphatic filariasis control in Tanzania: effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission.PLoS Negl Trop Dis. 2010 Jun 1;4(6):e696. doi: 10.1371/journal.pntd.0000696. PLoS Negl Trop Dis. 2010. PMID: 20532226 Free PMC article.
-
Molecular epidemiology, phylogeny and evolution of the filarial nematode Wuchereria bancrofti.Infect Genet Evol. 2014 Dec;28:33-43. doi: 10.1016/j.meegid.2014.08.018. Epub 2014 Aug 29. Infect Genet Evol. 2014. PMID: 25176600 Free PMC article. Review.
References
-
- Michael E, Bundy DA. Global mapping of lymphatic filariasis. Parasitol Today. 1997;13:472–476. - PubMed
-
- Durrheim DN, Wynd S, Liese B, Gyapong JO. Editorial: Lymphatic filariasis endemicity–an indicator of poverty? Trop Med Int Health. 2004;9:843–845. - PubMed
-
- Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. - DOI - PMC - PubMed
-
- Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Health. 2000;5:591–594. - PubMed
-
- Molyneux DH, Zagaria N. Lymphatic filariasis elimination: progress in global programme development. Ann Trop Med Parasitol. 2002;96(Suppl 2):S15–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

