Identification of novel glial genes by single-cell transcriptional profiling of Bergmann glial cells from mouse cerebellum
- PMID: 20169146
- PMCID: PMC2820553
- DOI: 10.1371/journal.pone.0009198
Identification of novel glial genes by single-cell transcriptional profiling of Bergmann glial cells from mouse cerebellum
Abstract
Bergmann glial cells play critical roles in the structure and function of the cerebellum. During development, their radial processes serve as guides for migrating granule neurons and their terminal endfeet tile to form the glia limitans. As the cerebellum matures, Bergmann glia perform important roles in synaptic transmission and synapse maintenance, while continuing to serve as essential structural elements. Despite growing evidence of the diverse functions of Bergmann glia, the molecular mechanisms that mediate these functions have remained largely unknown. As a step toward identifying the molecular repertoire underlying Bergmann glial function, here we examine global gene expression in individual Bergmann glia from developing (P6) and mature (P30) mouse cerebellum. When we select for developmentally regulated genes, we find that transcription factors and ribosomal genes are particularly enriched at P6 relative to P30; whereas synapse associated molecules are enriched at P30 relative to P6. We also analyze genes expressed at high levels at both ages. In all these categories, we find genes that were not previously known to be expressed in glial cells, and discuss novel functions some of these genes may potentially play in Bergmann glia. We also show that Bergmann glia, even in the adult, express a large set of genes thought to be specific to stem cells, suggesting that Bergmann glia may retain neural precursor potential as has been proposed. Finally, we highlight several genes that in the cerebellum are expressed in Bergmann glia but not astrocytes, and may therefore serve as new, specific markers for Bergmann glia.
Conflict of interest statement
Figures
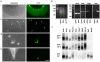
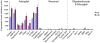

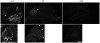
References
-
- Hausmann B, Sievers J. Cerebellar external granule cells are attached to the basal lamina from the onset of migration up to the end of their proliferative activity. J Comp Neurol. 1985;241:50–62. - PubMed
-
- Sievers J, Mangold U, Berry M, Allen C, Schlossberger HG. Experimental studies on cerebellar foliation. I. A qualitative morphological analysis of cerebellar fissuration defects after neonatal treatment with 6-OHDA in the rat. J Comp Neurol. 1981;203:751–769. - PubMed
-
- Sievers J, von Knebel Doeberitz C, Pehlemann FW, Berry M. Meningeal cells influence cerebellar development over a critical period. Anat Embryol (Berl) 1986;175:91–100. - PubMed
-
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. - PubMed
-
- Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

