High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy
- PMID: 20169160
- PMCID: PMC2821405
- DOI: 10.1371/journal.pone.0009221
High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy
Abstract
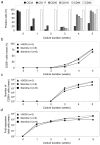
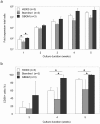
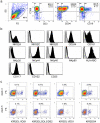
Immunotherapy based on natural killer (NK) cell infusions is a potential adjuvant treatment for many cancers. Such therapeutic application in humans requires large numbers of functional NK cells that have been selected and expanded using clinical grade protocols. We established an extremely efficient cytokine-based culture system for ex vivo expansion of NK cells from hematopoietic stem and progenitor cells from umbilical cord blood (UCB). Systematic refinement of this two-step system using a novel clinical grade medium resulted in a therapeutically applicable cell culture protocol. CD56(+)CD3(-) NK cell products could be routinely generated from freshly selected CD34(+) UCB cells with a mean expansion of >15,000 fold and a nearly 100% purity. Moreover, our protocol has the capacity to produce more than 3-log NK cell expansion from frozen CD34(+) UCB cells. These ex vivo-generated cell products contain NK cell subsets differentially expressing NKG2A and killer immunoglobulin-like receptors. Furthermore, UCB-derived CD56(+) NK cells generated by our protocol uniformly express high levels of activating NKG2D and natural cytotoxicity receptors. Functional analysis showed that these ex vivo-generated NK cells efficiently target myeloid leukemia and melanoma tumor cell lines, and mediate cytolysis of primary leukemia cells at low NK-target ratios. Our culture system exemplifies a major breakthrough in producing pure NK cell products from limited numbers of CD34(+) cells for cancer immunotherapy.
Conflict of interest statement
Figures



Similar articles
-
Frozen cord blood hematopoietic stem cells differentiate into higher numbers of functional natural killer cells in vitro than mobilized hematopoietic stem cells or freshly isolated cord blood hematopoietic stem cells.PLoS One. 2014 Jan 29;9(1):e87086. doi: 10.1371/journal.pone.0087086. eCollection 2014. PLoS One. 2014. PMID: 24489840 Free PMC article.
-
Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process.PLoS One. 2011;6(6):e20740. doi: 10.1371/journal.pone.0020740. Epub 2011 Jun 16. PLoS One. 2011. PMID: 21698239 Free PMC article.
-
A novel method to expand large numbers of CD56(+) natural killer cells from a minute fraction of selectively accessed cryopreserved cord blood for immunotherapy after transplantation.Cytotherapy. 2015 Nov;17(11):1582-93. doi: 10.1016/j.jcyt.2015.07.020. Cytotherapy. 2015. PMID: 26432560 Free PMC article.
-
Cytotoxic function of umbilical cord blood natural killer cells: relevance to adoptive immunotherapy.Pediatr Hematol Oncol. 2011 Nov;28(8):640-6. doi: 10.3109/08880018.2011.613092. Epub 2011 Oct 4. Pediatr Hematol Oncol. 2011. PMID: 21970456 Review.
-
The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells.Br J Haematol. 2009 Oct;147(2):185-91. doi: 10.1111/j.1365-2141.2009.07768.x. Br J Haematol. 2009. PMID: 19796267 Free PMC article. Review.
Cited by
-
Overcoming the UCB HSCs -Derived NK cells Dysfunction through Harnessing RAS/MAPK, IGF-1R and TGF-β Signaling Pathways.Cancer Cell Int. 2021 Jun 7;21(1):298. doi: 10.1186/s12935-021-01983-z. Cancer Cell Int. 2021. PMID: 34098947 Free PMC article.
-
Natural killer cells in clinical development as non-engineered, engineered, and combination therapies.J Hematol Oncol. 2022 Nov 8;15(1):164. doi: 10.1186/s13045-022-01382-5. J Hematol Oncol. 2022. PMID: 36348457 Free PMC article. Review.
-
Human NK cells: From development to effector functions.Innate Immun. 2021 Apr;27(3):212-229. doi: 10.1177/17534259211001512. Epub 2021 Mar 24. Innate Immun. 2021. PMID: 33761782 Free PMC article. Review.
-
Natural Killer Cell Immunotherapy: From Bench to Bedside.Front Immunol. 2015 Jun 3;6:264. doi: 10.3389/fimmu.2015.00264. eCollection 2015. Front Immunol. 2015. PMID: 26089820 Free PMC article. Review.
-
Human placental hematopoietic stem cell derived natural killer cells (CYNK-001) mediate protection against influenza a viral infection.Hum Vaccin Immunother. 2022 Nov 30;18(5):2055945. doi: 10.1080/21645515.2022.2055945. Epub 2022 Apr 11. Hum Vaccin Immunother. 2022. PMID: 35404743 Free PMC article.
References
-
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76(12):2421–2438. - PubMed
-
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–1519. - PubMed
-
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7(5):329–339. - PubMed
-
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. - PubMed
-
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

