On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from Bonobos
- PMID: 20169187
- PMCID: PMC2820532
- DOI: 10.1371/journal.ppat.1000765
On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from Bonobos
Abstract
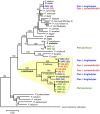
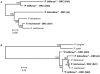
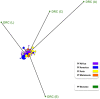
The origin of Plasmodium falciparum, the etiological agent of the most dangerous forms of human malaria, remains controversial. Although investigations of homologous parasites in African Apes are crucial to resolve this issue, studies have been restricted to a chimpanzee parasite related to P. falciparum, P. reichenowi, for which a single isolate was available until very recently. Using PCR amplification, we detected Plasmodium parasites in blood samples from 18 of 91 individuals of the genus Pan, including six chimpanzees (three Pan troglodytes troglodytes, three Pan t. schweinfurthii) and twelve bonobos (Pan paniscus). We obtained sequences of the parasites' mitochondrial genomes and/or from two nuclear genes from 14 samples. In addition to P. reichenowi, three other hitherto unknown lineages were found in the chimpanzees. One is related to P. vivax and two to P. falciparum that are likely to belong to distinct species. In the bonobos we found P. falciparum parasites whose mitochondrial genomes indicated that they were distinct from those present in humans, and another parasite lineage related to P. malariae. Phylogenetic analyses based on this diverse set of Plasmodium parasites in African Apes shed new light on the evolutionary history of P. falciparum. The data suggested that P. falciparum did not originate from P. reichenowi of chimpanzees (Pan troglodytes), but rather evolved in bonobos (Pan paniscus), from which it subsequently colonized humans by a host-switch. Finally, our data and that of others indicated that chimpanzees and bonobos maintain malaria parasites, to which humans are susceptible, a factor of some relevance to the renewed efforts to eradicate malaria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Qari SH, Shi Y-P, Pieniazek NJ, Collins WE, Lal AA. Phylogenetic relationship among the malaria parasites based on small subunit rRNA gene sequences: monophyletic nature of the human malaria parasite, Plasmodium falciparum. Mol Phylogenet Evol. 1996;6:157–165. - PubMed
-
- Escalante AA, Barrio E, Ayala FJ. Evolutionary origin of human and primate malarias: evidence from the circumsporozoite protein gene. Mol Biol Evol. 1995;12:616–626. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

