A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen
- PMID: 20169192
- PMCID: PMC2820538
- DOI: 10.1371/journal.pone.0009188
A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen
Abstract
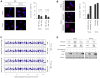
Cellular senescence irreversibly arrests cell proliferation in response to oncogenic stimuli. Human cells develop a senescence-associated secretory phenotype (SASP), which increases the secretion of cytokines and other factors that alter the behavior of neighboring cells. We show here that "senescent" mouse fibroblasts, which arrested growth after repeated passage under standard culture conditions (20% oxygen), do not express a human-like SASP, and differ from similarly cultured human cells in other respects. However, when cultured in physiological (3%) oxygen and induced to senesce by radiation, mouse cells more closely resemble human cells, including expression of a robust SASP. We describe two new aspects of the human and mouse SASPs. First, cells from both species upregulated the expression and secretion of several matrix metalloproteinases, which comprise a conserved genomic cluster. Second, for both species, the ability to promote the growth of premalignant epithelial cells was due primarily to the conserved SASP factor CXCL-1/KC/GRO-alpha. Further, mouse fibroblasts made senescent in 3%, but not 20%, oxygen promoted epithelial tumorigenesis in mouse xenographs. Our findings underscore critical mouse-human differences in oxygen sensitivity, identify conditions to use mouse cells to model human cellular senescence, and reveal novel conserved features of the SASP.
Conflict of interest statement
Figures




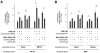

References
-
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. - PubMed
-
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. - PubMed
-
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Rev Molec Cell Biol. 2007;8:729–740. - PubMed
-
- Prieur A, S PD. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol. 2008;20:150–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

