Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice
- PMID: 20169203
- PMCID: PMC2820550
- DOI: 10.1371/journal.pone.0009192
Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice
Abstract
Background: Escherichia coli are widespread in the environment and pathogenic strains cause diseases of mucosal surfaces including the female genital tract. Pelvic inflammatory disease (PID; metritis) or endometritis affects approximately 40% of cattle after parturition. We tested the expectation that multiple genetically diverse E. coli from the environment opportunistically contaminate the uterine lumen after parturition to establish PID.
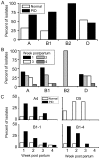
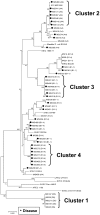
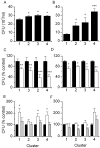
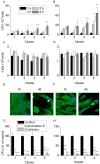
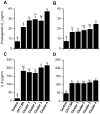
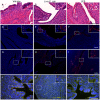
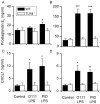
Methodology/principal findings: Distinct clonal groups of E. coli were identified by Random Amplification of Polymorphic DNA (RAPD) and Multilocus sequence typing (MLST) from animals with uterine disease and these differed from known diarrhoeic or extra-intestinal pathogenic E. coli. The endometrial pathogenic E. coli (EnPEC) were more adherent and invasive for endometrial epithelial and stromal cells, compared with E. coli isolated from the uterus of clinically unaffected animals. The endometrial epithelial and stromal cells produced more prostaglandin E(2) and interleukin-8 in response to lipopolysaccharide (LPS) purified from EnPEC compared with non-pathogenic E. coli. The EnPEC or their LPS also caused PID when infused into the uterus of mice with accumulation of neutrophils and macrophages in the endometrium. Infusion of EnPEC was only associated with bacterial invasion of the endometrium and myometrium. Despite their ability to invade cultured cells, elicit host cell responses and establish PID, EnPEC lacked sixteen genes commonly associated with adhesion and invasion by enteric or extraintestinal pathogenic E. coli, though the ferric yersiniabactin uptake gene (fyuA) was present in PID-associated EnPEC. Endometrial epithelial or stromal cells from wild type but not Toll-like receptor 4 (TLR4) null mice secreted prostaglandin E(2) and chemokine (C-X-C motif) ligand 1 (CXCL1) in response to LPS from EnPEC, highlighting the key role of LPS in PID.
Conclusions/significance: The implication arising from the discovery of EnPEC is that development of treatments or vaccines for PID should focus specifically on EnPEC and not other strains of E. coli.
Conflict of interest statement
Figures
References
-
- Deb K, Chaturvedi MM, Jaiswal YK. Comprehending the role of LPS in Gram-negative bacterial vaginosis: ogling into the causes of unfulfilled child-wish. Archives of Gynecology and Obstetrics. 2004;270:133–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

