Changes in plasma membrane structure and electromotile properties in prestin deficient outer hair cells
- PMID: 20169529
- PMCID: PMC2842980
- DOI: 10.1002/cm.20423
Changes in plasma membrane structure and electromotile properties in prestin deficient outer hair cells
Abstract
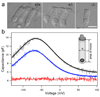
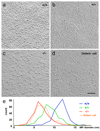
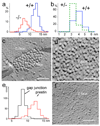
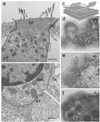
Cochlear outer hair cells (OHCs) rapidly change their length and stiffness when their membrane potential is altered. Prestin, the motor protein for this electromotility, is present along the OHC lateral plasma membrane where there is a high density of intra-membrane protein particles (IMPs). However, it is not known to what extent prestin contributes to this unusual dense population of proteins and overall organization of the membrane to generate the unique electromechanical response of OHCs. We investigated the relationship of prestin with the IMPs, the underlying cortical cytoskeletal lattice, and electromotility in prestin-deficient mice. Using freeze-fracture, we observed a reduction in density and size of the IMPs that correlates with the reduction and absence of prestin in the heterozygous and homozygous mice, respectively. We also observed a reduction or absence of electromotility-related charge density, axial stiffness, and piezoelectric properties of the OHC. A comparison of the charge density with the number of IMPs suggests that prestin forms tetramers in the wild type but is likely to form lower number oligomers in the prestin-deficient OHCs from the heterozygous mice. Interestingly, the characteristic actin-based cortical cytoskeletal lattice that underlies the membrane is absent in the prestin-null OHCs, suggesting that prestin is also required for recruiting or maintaining the cortical cytoskeletal lattice. These results suggest that the majority of the IMPs are indeed prestin and that electrically evoked length and stiffness changes are interrelated and dependent on both prestin and on the cortical actin cytoskeletal lattice of the OHC lateral membrane.
Figures


Similar articles
-
Functional prestin transduction of immature outer hair cells from normal and prestin-null mice.J Assoc Res Otolaryngol. 2008 Sep;9(3):307-20. doi: 10.1007/s10162-008-0121-3. Epub 2008 May 28. J Assoc Res Otolaryngol. 2008. PMID: 18506528 Free PMC article.
-
Prestin and electromotility may serve multiple roles in cochlear outer hair cells.Hear Res. 2022 Sep 15;423:108428. doi: 10.1016/j.heares.2021.108428. Epub 2021 Dec 26. Hear Res. 2022. PMID: 34987016 Review.
-
Prestin is expressed on the whole outer hair cell basolateral surface.Brain Res. 2006 Jun 20;1095(1):51-8. doi: 10.1016/j.brainres.2006.04.017. Epub 2006 May 18. Brain Res. 2006. PMID: 16709400 Free PMC article.
-
Activity-dependent regulation of prestin expression in mouse outer hair cells.J Neurophysiol. 2015 Jun 1;113(10):3531-42. doi: 10.1152/jn.00869.2014. Epub 2015 Mar 25. J Neurophysiol. 2015. PMID: 25810486 Free PMC article.
-
Outer hair cell electromechanics as a problem in soft matter physics: Prestin, the membrane and the cytoskeleton.Hear Res. 2022 Sep 15;423:108426. doi: 10.1016/j.heares.2021.108426. Epub 2021 Dec 31. Hear Res. 2022. PMID: 35101286 Review.
Cited by
-
Normal hearing sensitivity at low-to-middle frequencies with 34% prestin-charge density.PLoS One. 2012;7(9):e45453. doi: 10.1371/journal.pone.0045453. Epub 2012 Sep 21. PLoS One. 2012. PMID: 23029017 Free PMC article.
-
Characterization of Hair Cell-Like Cells Converted From Supporting Cells After Notch Inhibition in Cultures of the Organ of Corti From Neonatal Gerbils.Front Cell Neurosci. 2018 Mar 20;12:73. doi: 10.3389/fncel.2018.00073. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29662441 Free PMC article.
-
Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea.Compr Physiol. 2017 Sep 12;7(4):1197-1227. doi: 10.1002/cphy.c160049. Compr Physiol. 2017. PMID: 28915323 Free PMC article. Review.
-
The pathogenic roles of the p.R130S prestin variant in DFNB61 hearing loss.J Physiol. 2024 Mar;602(6):1199-1210. doi: 10.1113/JP285599. Epub 2024 Mar 3. J Physiol. 2024. PMID: 38431907 Free PMC article.
-
Evidence that prestin has at least two voltage-dependent steps.J Biol Chem. 2011 Jan 21;286(3):2297-307. doi: 10.1074/jbc.M110.185694. Epub 2010 Nov 11. J Biol Chem. 2011. PMID: 21071769 Free PMC article.
References
-
- Adler HJ, Belyantseva IA, Merritt RC, Jr, Frolenkov GI, Dougherty GW, Kachar B. Expression of prestin, a membrane motor protein, in the mammalian auditory and vestibular periphery. Hear Res. 2003;184(1–2):27–40. - PubMed
-
- Ashmore JF. In: Transducer motor coupling in cochlear outer hair cells. Kemp D, Wilson JP, editors. New York: Plenum Press; 1989.
-
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227(4683):194–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

