Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia
- PMID: 20169535
- PMCID: PMC9134828
- DOI: 10.1002/cm.20428
Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia
Abstract
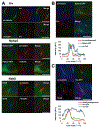
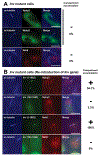
A primary cilium is an antenna-like structure extending from the surface of most vertebrate cells. It is structurally divided along its vertical axis into sub-compartments that include the ciliary tip, the shaft, the ciliary necklace segment, the transitional zone and the basal body. We recently discovered that the shaft of the primary cilia has a distinct molecular compartment, termed the "Inv compartment", which is characterized by the accumulation of Inv at the base of primary cilia. Inv was discovered as a causative gene in inv mutant mice. It was later found to be responsible for the infantile type of nephronophthisis (NPHP2). Nephronophthisis (NPHP) is an autosomal recessive kidney disease. Nine causative genes have been identified, with all examined products thought to function in cilia, basal body and/or centrioles. However, their exact intra-ciliary localization and relationship have not been clear. Here, we report that products of Nphp3 and Nek8 (the mouse orthologs of the causative genes for NPHP3 and NPHP9, respectively) localize to the Inv compartment. We also show that Inv is essential for the compartmental localization of Nphp3 and Nek8, whereas localization of Inv does not require Nphp3 or Nek8. Nphp1 and Nphp4 also localize at the proximal region of the cilium, but not in Inv compartment. Our results indicate that Inv acts as an anchor for Nphp3 and Nek8 in the Inv compartment, and suggest that Inv compartment is a candidate site for intra-ciliary interaction of Inv, Nphp3 and Nek8.
2010 Wiley-Liss, Inc.
Figures

Similar articles
-
The ciliary protein Nek8/Nphp9 acts downstream of Inv/Nphp2 during pronephros morphogenesis and left-right establishment in zebrafish.FEBS Lett. 2012 Jul 30;586(16):2273-9. doi: 10.1016/j.febslet.2012.05.064. Epub 2012 Jun 8. FEBS Lett. 2012. PMID: 22687244
-
The Inv compartment of renal cilia is an intraciliary signal-activating center to phosphorylate ANKS6.Kidney Int. 2018 May;93(5):1108-1117. doi: 10.1016/j.kint.2017.11.016. Epub 2018 Feb 1. Kidney Int. 2018. PMID: 29395339
-
ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3.Nat Genet. 2013 Aug;45(8):951-6. doi: 10.1038/ng.2681. Epub 2013 Jun 23. Nat Genet. 2013. PMID: 23793029 Free PMC article.
-
NEK8, a NIMA-family protein kinase at the core of the ciliary INV complex.Cell Commun Signal. 2025 Apr 7;23(1):170. doi: 10.1186/s12964-025-02143-w. Cell Commun Signal. 2025. PMID: 40189576 Free PMC article. Review.
-
The ciliary transitional zone and nephrocystins.Differentiation. 2012 Feb;83(2):S91-6. doi: 10.1016/j.diff.2011.11.006. Epub 2011 Dec 12. Differentiation. 2012. PMID: 22169048 Review.
Cited by
-
Cystic kidney diseases: many ways to form a cyst.Pediatr Nephrol. 2013 Jan;28(1):33-49. doi: 10.1007/s00467-012-2221-x. Epub 2012 Jun 27. Pediatr Nephrol. 2013. PMID: 22736301 Free PMC article. Review.
-
NPHP proteins are binding partners of nucleoporins at the base of the primary cilium.PLoS One. 2019 Sep 25;14(9):e0222924. doi: 10.1371/journal.pone.0222924. eCollection 2019. PLoS One. 2019. PMID: 31553752 Free PMC article.
-
Dimerization activates the Inversin complex in C. elegans.Mol Biol Cell. 2024 Oct 1;35(10):ar127. doi: 10.1091/mbc.E24-05-0218. Epub 2024 Aug 7. Mol Biol Cell. 2024. PMID: 39110529 Free PMC article.
-
The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis.Hum Mol Genet. 2012 Mar 1;21(5):1155-71. doi: 10.1093/hmg/ddr544. Epub 2011 Nov 21. Hum Mol Genet. 2012. PMID: 22106379 Free PMC article.
-
High-resolution genetic localization of a modifying locus affecting disease severity in the juvenile cystic kidneys (jck) mouse model of polycystic kidney disease.Mamm Genome. 2016 Jun;27(5-6):191-9. doi: 10.1007/s00335-016-9633-z. Epub 2016 Apr 25. Mamm Genome. 2016. PMID: 27114383 Free PMC article.
References
-
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, Ozyurek H, Farin FM, Kroes HY, Wolfrum U, Brunner HG, Cremers FP, Glass IA, Knoers NV, Roepman R. 2007. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet 39(7):882–888. - PubMed
-
- Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, Seelow D, Nurnberg G, Becker C, Chudley AE, Nurnberg P, Hildebrandt F, Treier M. 2007. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet 39(8):1018–1024. - PubMed
-
- Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, Nurnberg G, Becker C, Grimm T, Girschick G, Lynch SA, Kelehan P, Senderek J, Neuhaus TJ, Stallmach T, Zentgraf H, Nurnberg P, Gretz N, Lo C, Lienkamp S, Schafer T, Walz G, Benzing T, Zerres K, Omran H. 2008. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 82(4): 959–970. - PMC - PubMed
-
- Bowers AJ, Boylan JF. 2004. Nek8, a NIMA family kinase member, is over-expressed in primary human breast tumors. Gene 328:135–142. - PubMed
-
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. 2001. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol 11(20): 1586–1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

