Conversion of methionine into homocysteic acid in heavily oxidized proteomics samples
- PMID: 20169556
- PMCID: PMC2935272
- DOI: 10.1002/rcm.4447
Conversion of methionine into homocysteic acid in heavily oxidized proteomics samples
Abstract
Analysis of protein oxidation is necessary in numerous areas of biochemistry, including hydroxyl radical surface mapping, oxidative stress assays, and pharmaceutical stability testing. Mass spectrometry is one of the tools most often used to identify protein oxidation products, and previous studies have attempted to identify and characterize all of the major oxidation products detected by mass spectrometry for each amino acid residue. In this note, we present evidence that in heavily oxidized protein samples, such as those produced by hydroxyl radical surface mapping, a major oxidation product of methionine is homocysteic acid. The formation of homocysteic acid from methionine was previously unrecognized in other mass spectrometric analyses, and has important implications for the analysis of oxidized samples, as well as potential implications as to the functional consequences of methionine oxidation.
Copyright (c) 2010 John Wiley & Sons, Ltd.
Figures
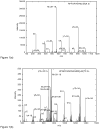
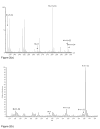

References
-
- Xu G, Chance MR. Chem Rev. 2007;107:3514–3543. - PubMed
-
- Xu G, Chance MR. Anal Chem. 2005;77:4549–4555. - PubMed
-
- Bern M, Cai Y, Goldberg D. Anal Chem. 2007;79:1393–1400. - PubMed
-
- Kopoldova J, Kolousek J, Babicky A, Liebster J. Nature. 1958;182:1074–1076. - PubMed
-
- Ohara A. J Radiat Res (Tokyo) 1966;7:18–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
