Lipid peroxyl radicals mediate tyrosine dimerization and nitration in membranes
- PMID: 20170094
- PMCID: PMC3506709
- DOI: 10.1021/tx900446r
Lipid peroxyl radicals mediate tyrosine dimerization and nitration in membranes
Abstract
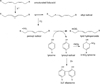
Protein tyrosine dimerization and nitration by biologically relevant oxidants usually depend on the intermediate formation of tyrosyl radical ((*)Tyr). In the case of tyrosine oxidation in proteins associated with hydrophobic biocompartments, the participation of unsaturated fatty acids in the process must be considered since they typically constitute preferential targets for the initial oxidative attack. Thus, we postulate that lipid-derived radicals mediate the one-electron oxidation of tyrosine to (*)Tyr, which can afterward react with another (*)Tyr or with nitrogen dioxide ((*)NO(2)) to yield 3,3'-dityrosine or 3-nitrotyrosine within the hydrophobic structure, respectively. To test this hypothesis, we have studied tyrosine oxidation in saturated and unsaturated fatty acid-containing phosphatidylcholine (PC) liposomes with an incorporated hydrophobic tyrosine analogue BTBE (N-t-BOC l-tyrosine tert-butyl ester) and its relationship with lipid peroxidation promoted by three oxidation systems, namely, peroxynitrite, hemin, and 2,2'-azobis (2-amidinopropane) hydrochloride. In all cases, significant tyrosine (BTBE) oxidation was seen in unsaturated PC liposomes, in a way that was largely decreased at low oxygen concentrations. Tyrosine oxidation levels paralleled those of lipid peroxidation (i.e., malondialdehyde and lipid hydroperoxides), lipid-derived radicals and BTBE phenoxyl radicals were simultaneously detected by electron spin resonance spin trapping, supporting an association between the two processes. Indeed, alpha-tocopherol, a known reactant with lipid peroxyl radicals (LOO(*)), inhibited both tyrosine oxidation and lipid peroxidation induced by all three oxidation systems. Moreover, oxidant-stimulated liposomal oxygen consumption was dose dependently inhibited by BTBE but not by its phenylalanine analogue, BPBE (N-t-BOC l-phenylalanine tert-butyl ester), providing direct evidence for the reaction between LOO(*) and the phenol moiety in BTBE, with an estimated second-order rate constant of 4.8 x 10(3) M(-1) s(-1). In summary, the data presented herein demonstrate that LOO(*) mediates tyrosine oxidation processes in hydrophobic biocompartments and provide a new mechanistic insight to understand protein oxidation and nitration in lipoproteins and biomembranes.
Figures
















References
-
- Bodaness RS, Leclair M, Zigler JS., Jr An analysis of the H2O2-mediated crosslinking of lens crystallins catalyzed by the heme-undecapeptide from cytochrome c. Arch Biochem Biophys. 1984;231:461–469. - PubMed
-
- Giulivi C, Traaseth NJ, Davies KJ. Tyrosine oxidation products: analysis and biological relevance. Amino Acids. 2003;25:227–232. - PubMed
-
- Heinecke JW. Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins. Toxicology. 2002;177:11–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

