Molecular gastronomy: a new emerging scientific discipline
- PMID: 20170128
- PMCID: PMC2855180
- DOI: 10.1021/cr900105w
Molecular gastronomy: a new emerging scientific discipline
Figures

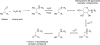
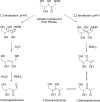
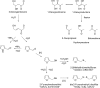
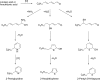
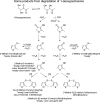
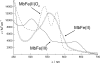

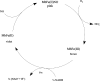
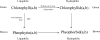
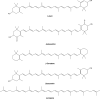
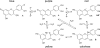
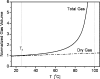
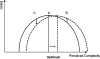
References
-
- Slavin H. C. J. Am. Dental Assoc. 1999, 130, 1497–1500. - PubMed
-
- van der Linden E.; McClements D. J.; Ubbink J. Food Biophys. 2008, 3, 246–254.
-
- Vega C.; Ubbink J. Trends Food Sci. Technol. 2008, 19, 372–382.
-
- World’s 50 best restuarants. http://www.theworlds50best.com/2008_list.html, 2008.
-
- Think Books. World’s Best Restaurants; Pan MacMillan: London, 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

