Assessment of the efficacy of commercially available and candidate vaccines against a pandemic H1N1 2009 virus
- PMID: 20170374
- PMCID: PMC7109664
- DOI: 10.1086/651171
Assessment of the efficacy of commercially available and candidate vaccines against a pandemic H1N1 2009 virus
Abstract
Background: The emergence and global spread of the pandemic H1N1 2009 influenza virus have raised questions regarding the protective effect of available seasonal vaccines and the efficacy of a newly produced matched vaccine.
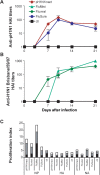
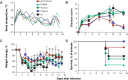
Methods: Ferrets were immunized with the 2008-2009 formulations of commercially available live attenuated (FluMist; MedImmune) or split-inactivated (Fluviral; GlaxoSmithKline) vaccines, a commercial swine vaccine (FluSure; Pfizer), or a laboratory-produced matched inactivated whole-virus vaccine (A/Mexico/InDRE4487/2009). Adaptive immune responses were monitored, and the animals were challenged with A/Mexico/InDRE4487/2009 after 5 weeks.
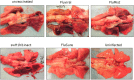
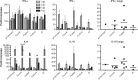
Results: Only animals that received the swine or matched vaccines developed detectable hemagglutination-inhibiting antibodies against the challenge virus, whereas a T cell response was exclusively detected in animals vaccinated with FluMist. After challenge, all animals had high levels of virus replication in the upper respiratory tract. However, preexisting anti-pandemic H1N1 2009 antibodies resulted in reduced clinical signs and improved survival. Surprisingly, FluMist was associated with a slight increase in mortality and greater lung damage, which correlated with early up-regulation of interleukin-10.
Conclusions: The present study demonstrates that a single dose of matched inactivated vaccine confers partial protection against a pandemic H1N1 2009 virus, and it suggests that a higher dose or prime-boost regimen may be required. The consequences of mismatched immunity to influenza merit further investigation.
Figures

References
-
- World Health Organization (WHO) Pandemic (H1N1) 2009-update 60. Geneva, Switzerland: WHO, 31 July 2009.
-
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. - PubMed
-
- Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

