Immune signatures in human PBMCs of idiotypic vaccine for HCV-related lymphoproliferative disorders
- PMID: 20170491
- PMCID: PMC2839974
- DOI: 10.1186/1479-5876-8-18
Immune signatures in human PBMCs of idiotypic vaccine for HCV-related lymphoproliferative disorders
Abstract
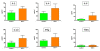
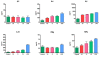
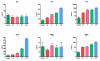
Hepatitis C virus (HCV) is one of the major risk factors for chronic hepatitis, which may progress to cirrhosis and hepatocellular carcinoma, as well as for type II mixed cryoglobulinemia (MC), which may further evolve into an overt B-cell non-Hodgkin's lymphoma (NHL). It has been previously shown that B-cell receptor (BCR) repertoire, expressed by clonal B-cells involved in type II MC as well as in HCV-associated NHL, is constrained to a limited number of variable heavy (VH)- and light (VL)-chain genes. Among these, the VK3-20 light chain idiotype has been selected as a possible target for passive as well as active immunization strategy. In the present study, we describe the results of a multiparametric analysis of the innate and early adaptive immune response after ex vivo stimulation of human immune cells with the VK3-20 protein. This objective has been pursued by implementing high-throughput technologies such as multiparameter flow cytometry and multiplex analysis of cytokines and chemokines.
Figures






Similar articles
-
HCV proteins and immunoglobulin variable gene (IgV) subfamilies in HCV-induced type II mixed cryoglobulinemia: a concurrent pathogenetic role.Clin Dev Immunol. 2012;2012:705013. doi: 10.1155/2012/705013. Epub 2012 May 29. Clin Dev Immunol. 2012. PMID: 22690241 Free PMC article. Review.
-
Multiparametric analyses of human PBMCs loaded ex vivo with a candidate idiotype vaccine for HCV-related lymphoproliferative disorders.PLoS One. 2012;7(9):e44870. doi: 10.1371/journal.pone.0044870. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028651 Free PMC article.
-
Characterization of antibodies directed against the immunoglobulin light kappa chain variable chain region (VK) of hepatitis C virus-related type-II mixed cryoglobulinemia and B-cell proliferations.Ann N Y Acad Sci. 2009 Sep;1173:152-60. doi: 10.1111/j.1749-6632.2009.04677.x. Ann N Y Acad Sci. 2009. PMID: 19758144
-
Hepatitis C virus infection and cryoglobulinaemia.Forum (Genova). 1998 Jan-Mar;8(1):95-103. Forum (Genova). 1998. PMID: 9514994 Review.
-
Induction of interleukin-6 by hepatitis C virus core protein in hepatitis C-associated mixed cryoglobulinemia and B-cell non-Hodgkin's lymphoma.Clin Cancer Res. 2006 Aug 1;12(15):4491-8. doi: 10.1158/1078-0432.CCR-06-0154. Clin Cancer Res. 2006. PMID: 16899594
Cited by
-
HCV proteins and immunoglobulin variable gene (IgV) subfamilies in HCV-induced type II mixed cryoglobulinemia: a concurrent pathogenetic role.Clin Dev Immunol. 2012;2012:705013. doi: 10.1155/2012/705013. Epub 2012 May 29. Clin Dev Immunol. 2012. PMID: 22690241 Free PMC article. Review.
-
Prediction of individual immune responsiveness to a candidate vaccine by a systems vaccinology approach.J Transl Med. 2014 Jan 15;12:11. doi: 10.1186/1479-5876-12-11. J Transl Med. 2014. PMID: 24428943 Free PMC article.
-
Immunological effects of adjuvants in subsets of antigen presenting cells of cancer patients undergoing chemotherapy.J Transl Med. 2020 Jan 23;18(1):34. doi: 10.1186/s12967-020-02218-x. J Transl Med. 2020. PMID: 31973714 Free PMC article.
-
Multiparametric analyses of human PBMCs loaded ex vivo with a candidate idiotype vaccine for HCV-related lymphoproliferative disorders.PLoS One. 2012;7(9):e44870. doi: 10.1371/journal.pone.0044870. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028651 Free PMC article.
-
Translating tumor antigens into cancer vaccines.Clin Vaccine Immunol. 2011 Jan;18(1):23-34. doi: 10.1128/CVI.00286-10. Epub 2010 Nov 3. Clin Vaccine Immunol. 2011. PMID: 21048000 Free PMC article. Review.
References
-
- Ferri C, Longombardo G, La CL, Greco F, Lombardini F, Cecchetti R, Cagianelli MA, Marchi S, Monti M, Zignego AL. Hepatitis C virus chronic infection as a common cause of mixed cryoglobulinaemia and autoimmune liver disease. J Intern Med. 1994;236:31–36. doi: 10.1111/j.1365-2796.1994.tb01116.x. - DOI - PubMed
-
- Zuckerman E, Zuckerman T, Levine AM, Douer D, Gutekunst K, Mizokami M, Qian DG, Velankar M, Nathwani BN, Fong TL. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997;127:423–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

