Subolesin expression in response to pathogen infection in ticks
- PMID: 20170494
- PMCID: PMC2836984
- DOI: 10.1186/1471-2172-11-7
Subolesin expression in response to pathogen infection in ticks
Abstract
Background: Ticks (Acari: Ixodidae) are vectors of pathogens worldwide that cause diseases in humans and animals. Ticks and pathogens have co-evolved molecular mechanisms that contribute to their mutual development and survival. Subolesin was discovered as a tick protective antigen and was subsequently shown to be similar in structure and function to akirins, an evolutionarily conserved group of proteins in insects and vertebrates that controls NF-kB-dependent and independent expression of innate immune response genes. The objective of this study was to investigate subolesin expression in several tick species infected with a variety of pathogens and to determine the effect of subolesin gene knockdown on pathogen infection. In the first experiment, subolesin expression was characterized in ticks experimentally infected with the cattle pathogen, Anaplasma marginale. Subolesin expression was then characterized in questing or feeding adult ticks confirmed to be infected with Anaplasma, Ehrlichia, Rickettsia, Babesia or Theileria spp. Finally, the effect of subolesin knockdown by RNA interference (RNAi) on tick infection was analyzed in Dermacentor variabilis males exposed to various pathogens by capillary feeding (CF).
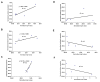
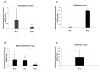
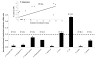
Results: Subolesin expression increased with pathogen infection in the salivary glands but not in the guts of tick vector species infected with A. marginale. When analyzed in whole ticks, subolesin expression varied between tick species and in response to different pathogens. As reported previously, subolesin knockdown in D. variabilis infected with A. marginale and other tick-borne pathogens resulted in lower infection levels, while infection with Francisella tularensis increased in ticks after RNAi. When non-tick-borne pathogens were fed to ticks by CF, subolesin RNAi did not affect or resulted in lower infection levels in ticks. However, subolesin expression was upregulated in D. variabilis exposed to Escherichia coli, suggesting that although this pathogen may induce subolesin expression in ticks, silencing of this molecule reduced bacterial multiplication by a presently unknown mechanism.
Conclusions: Subolesin expression in infected ticks suggested that subolesin may be functionally important for tick innate immunity to pathogens, as has been reported for the akirins. However, subolesin expression and consequently subolesin-mediated innate immunity varied with the pathogen and tick tissue. Subolesin may plays a role in tick innate immunity in the salivary glands by limiting pathogen infection levels, but activates innate immunity only for some pathogen in the guts and other tissues. In addition, these results provided additional support for the role of subolesin in other molecular pathways including those required for tissue development and function and for pathogen infection and multiplication in ticks. Consequently, RNAi experiments demonstrated that subolesin knockdown in ticks may affect pathogen infection directly by reducing tick innate immunity that results in higher infection levels and indirectly by affecting tissue structure and function and the expression of genes that interfere with pathogen infection and multiplication. The impact of the direct or indirect effects of subolesin knockdown on pathogen infection may depend on several factors including specific tick-pathogen molecular interactions, pathogen life cycle in the tick and unknown mechanisms affected by subolesin function in the control of global gene expression in ticks.
Figures
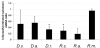
Similar articles
-
Differential expression of the tick protective antigen subolesin in anaplasma marginale- and A. phagocytophilum-infected host cells.Ann N Y Acad Sci. 2008 Dec;1149:27-35. doi: 10.1196/annals.1428.056. Ann N Y Acad Sci. 2008. PMID: 19120168
-
Targeting the tick protective antigen subolesin reduces vector infestations and pathogen infection by Anaplasma marginale and Babesia bigemina.Vaccine. 2011 Nov 3;29(47):8575-9. doi: 10.1016/j.vaccine.2011.09.023. Epub 2011 Sep 25. Vaccine. 2011. PMID: 21951878
-
Differential expression of genes in salivary glands of male Rhipicephalus (Boophilus)microplus in response to infection with Anaplasma marginale.BMC Genomics. 2010 Mar 18;11:186. doi: 10.1186/1471-2164-11-186. BMC Genomics. 2010. PMID: 20298599 Free PMC article.
-
Subolesin/Akirin vaccines for the control of arthropod vectors and vectorborne pathogens.Transbound Emerg Dis. 2013 Nov;60 Suppl 2:172-8. doi: 10.1111/tbed.12146. Transbound Emerg Dis. 2013. PMID: 24589118 Review.
-
Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts.Int J Mol Sci. 2020 Jul 30;21(15):5437. doi: 10.3390/ijms21155437. Int J Mol Sci. 2020. PMID: 32751625 Free PMC article. Review.
Cited by
-
Induction of humoral immune response to multiple recombinant Rhipicephalus appendiculatus antigens and their effect on tick feeding success and pathogen transmission.Parasit Vectors. 2016 Sep 2;9(1):484. doi: 10.1186/s13071-016-1774-0. Parasit Vectors. 2016. PMID: 27589998 Free PMC article.
-
Effect of Silencing subolesin and enolase impairs gene expression, engorgement and reproduction in Haemaphysalis longicornis (Acari: Ixodidae) ticks.J Vet Sci. 2024 May;25(3):e43. doi: 10.4142/jvs.24039. J Vet Sci. 2024. PMID: 38834512 Free PMC article.
-
Tick vaccines and the control of tick-borne pathogens.Front Cell Infect Microbiol. 2013 Jul 9;3:30. doi: 10.3389/fcimb.2013.00030. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23847771 Free PMC article. Review.
-
Reciprocal regulation of NF-kB (Relish) and Subolesin in the tick vector, Ixodes scapularis.PLoS One. 2013 Jun 12;8(6):e65915. doi: 10.1371/journal.pone.0065915. Print 2013. PLoS One. 2013. PMID: 23776567 Free PMC article.
-
Counterattacking the tick bite: towards a rational design of anti-tick vaccines targeting pathogen transmission.Parasit Vectors. 2019 May 14;12(1):229. doi: 10.1186/s13071-019-3468-x. Parasit Vectors. 2019. PMID: 31088506 Free PMC article. Review.
References
-
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51(Pt 6):2145–2165. - PubMed
-
- Kocan KM. Development of Anaplasma marginale in ixodid ticks: coordinated development of a rickettsial organism and its tick host. England: Ellis Horwood Ltd; 1986.
-
- Kocan KM, Stiller D, Goff WL, Claypool PL, Edwards W, Ewing SA, McGuire TC, Hair JA, Barron SJ. Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am J Vet Res. 1992;53(4):499–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

