SEL1L deficiency impairs growth and differentiation of pancreatic epithelial cells
- PMID: 20170518
- PMCID: PMC2848149
- DOI: 10.1186/1471-213X-10-19
SEL1L deficiency impairs growth and differentiation of pancreatic epithelial cells
Abstract
Background: The vertebrate pancreas contains islet, acinar and ductal cells. These cells derive from a transient pool of multipotent pancreatic progenitors during embryonic development. Insight into the genetic determinants regulating pancreatic organogenesis will help the development of cell-based therapies for the treatment of diabetes mellitus. Suppressor enhancer lin12/Notch 1 like (Sel1l) encodes a cytoplasmic protein that is highly expressed in the developing mouse pancreas. However, the morphological and molecular events regulated by Sel1l remain elusive.
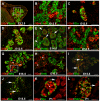
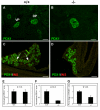
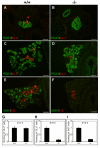
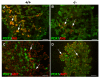
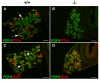
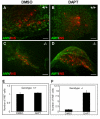
Results: We have characterized the pancreatic phenotype of mice carrying a gene trap mutation in Sel1l. We show that Sel1l expression in the developing pancreas coincides with differentiation of the endocrine and exocrine lineages. Mice homozygous for the gene trap mutation die prenatally and display an impaired pancreatic epithelial morphology and cell differentiation. The pancreatic epithelial cells of Sel1l mutant embryos are confined to the progenitor cell state throughout the secondary transition. Pharmacological inhibition of Notch signaling partially rescues the pancreatic phenotype of Sel1l mutant embryos.
Conclusions: Together, these data suggest that Sel1l is essential for the growth and differentiation of endoderm-derived pancreatic epithelial cells during mouse embryonic development.
Figures
Similar articles
-
The nuclear hormone receptor family member NR5A2 controls aspects of multipotent progenitor cell formation and acinar differentiation during pancreatic organogenesis.Development. 2014 Aug;141(16):3123-33. doi: 10.1242/dev.109405. Epub 2014 Jul 25. Development. 2014. PMID: 25063451 Free PMC article.
-
Mechanosignalling via integrins directs fate decisions of pancreatic progenitors.Nature. 2018 Dec;564(7734):114-118. doi: 10.1038/s41586-018-0762-2. Epub 2018 Nov 28. Nature. 2018. PMID: 30487608
-
Feedback control of growth, differentiation, and morphogenesis of pancreatic endocrine progenitors in an epithelial plexus niche.Genes Dev. 2015 Oct 15;29(20):2203-16. doi: 10.1101/gad.267914.115. Genes Dev. 2015. PMID: 26494792 Free PMC article.
-
Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells.Semin Cell Dev Biol. 2012 Aug;23(6):711-9. doi: 10.1016/j.semcdb.2012.06.008. Epub 2012 Jun 26. Semin Cell Dev Biol. 2012. PMID: 22743232 Free PMC article. Review.
-
Lineage commitment and cellular differentiation in exocrine pancreas.Pancreatology. 2001;1(6):587-96. doi: 10.1159/000055868. Pancreatology. 2001. PMID: 12120241 Review.
Cited by
-
mSEL-1L (Suppressor/enhancer Lin12-like) protein levels influence murine neural stem cell self-renewal and lineage commitment.J Biol Chem. 2011 May 27;286(21):18708-19. doi: 10.1074/jbc.M110.210740. Epub 2011 Mar 31. J Biol Chem. 2011. PMID: 21454627 Free PMC article.
-
Endoplasmic reticulum quality control in cancer: Friend or foe.Semin Cancer Biol. 2015 Aug;33:25-33. doi: 10.1016/j.semcancer.2015.02.003. Epub 2015 Mar 18. Semin Cancer Biol. 2015. PMID: 25794824 Free PMC article. Review.
-
Growth Arrest Specific 2 (GAS2) is a Critical Mediator of Germ Cell Cyst Breakdown and Folliculogenesis in Mice.Sci Rep. 2016 Oct 13;6:34956. doi: 10.1038/srep34956. Sci Rep. 2016. PMID: 27734842 Free PMC article.
-
Pancreas lineage allocation and specification are regulated by sphingosine-1-phosphate signalling.PLoS Biol. 2017 Mar 1;15(3):e2000949. doi: 10.1371/journal.pbio.2000949. eCollection 2017 Mar. PLoS Biol. 2017. PMID: 28248965 Free PMC article.
-
Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival.Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):E582-91. doi: 10.1073/pnas.1318114111. Epub 2014 Jan 22. Proc Natl Acad Sci U S A. 2014. PMID: 24453213 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

