Hepatitis B virus X protein upregulates HSP90alpha expression via activation of c-Myc in human hepatocarcinoma cell line, HepG2
- PMID: 20170530
- PMCID: PMC2841080
- DOI: 10.1186/1743-422X-7-45
Hepatitis B virus X protein upregulates HSP90alpha expression via activation of c-Myc in human hepatocarcinoma cell line, HepG2
Abstract
Background: The Hepatitis B Virus X protein (HBx) plays a major role in hepatocellular carcinoma (HCC) development, however, its contribution to tumor invasion and metastasis has not been established so far. Heat shock protein 90 alpha (HSP90alpha) isoform is an ATP-dependent molecular chaperone that maintains the active conformation of client oncoproteins in cancer cells, which is abundantly expressed in HCC, especially in hepatitis B virus (HBV)-related tumors, might be involved in tumor progression.
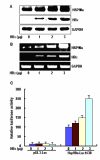
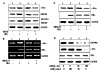
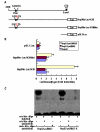
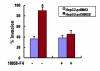
Methods: The levels of HSP90alpha, extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylated ERK1/2 (p-ERK1/2) and c-Myc in HBx-transfected HepG2 cells were determined by western blots analysis. The endogenous ERKs activity was demonstrated by ELISA assay. The regulation of c-Myc-mediated HSP90 alpha promoter transactivation by HBx was evaluated through electrophoretic mobility shift analysis (EMSA). The c-Myc-mediated HSP90alpha transcription was analysed by promoter assay. The HBx-expressing cells were transfected with specific small interference RNA (siRNA) against c-Myc. The in vitro invasion potentials of cells were evaluated by Transwell cell invasion assay.
Results: HBx induces HSP90alpha expression at the transcription level. The induction effect of HBx was inhibited after treatment with c-Myc inhibitor, 10058-F4. In addition, the luciferase activity of the HSP90alpha promoter analysis revealed that the HBx is directly involved in the c-Myc-mediated transcriptional activation of HSP90alpha. Furthermore, HBx induces c-Myc expression by activation of Ras/Raf/ERK1/2 cascades, which in turn results in activation of the c-Myc-mediated HSP90alpha promoter and subsequently up-regulation of the HSP90alpha expression. Overexpression of HSP90alpha in HBx-transfected cells enhances tumor cells invasion. siRNA-mediated c-Myc knockdown in HBx-transfected cells significantly suppressed HSP90alpha expression and cells invasion in vitro.
Conclusion: These results demonstrate the ability of HBx to promote tumor cells invasion by a mechanism involving the up-regulation of HSP90alpha and provide new insights into the mechanism of action of HBx and its involvement in tumor metastasis and recurrence of HCC.
Figures
Similar articles
-
Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential.FASEB J. 2004 Jul;18(10):1123-5. doi: 10.1096/fj.03-1429fje. Epub 2004 May 7. FASEB J. 2004. PMID: 15132991
-
Role of hepatitis B virus X protein in regulating LIM and SH3 protein 1 (LASP-1) expression to mediate proliferation and migration of hepatoma cells.Virol J. 2012 Aug 16;9:163. doi: 10.1186/1743-422X-9-163. Virol J. 2012. PMID: 22897902 Free PMC article.
-
MiR-19a, miR-122 and miR-223 are differentially regulated by hepatitis B virus X protein and involve in cell proliferation in hepatoma cells.J Transl Med. 2016 May 5;14(1):122. doi: 10.1186/s12967-016-0888-7. J Transl Med. 2016. PMID: 27150195 Free PMC article.
-
Hepatitis B virus X protein sensitizes UV-induced apoptosis by transcriptional transactivation of Fas ligand gene expression.IUBMB Life. 2005 Sep;57(9):651-8. doi: 10.1080/15216540500239697. IUBMB Life. 2005. PMID: 16203685 Review.
-
Extracellular heat shock protein 90 alpha (eHsp90α)'s role in cancer progression and the development of therapeutic strategies.Eur J Med Chem. 2024 Nov 5;277:116736. doi: 10.1016/j.ejmech.2024.116736. Epub 2024 Aug 2. Eur J Med Chem. 2024. PMID: 39126794 Review.
Cited by
-
A re-emerging marker for prognosis in hepatocellular carcinoma: the add-value of fishing c-myc gene for early relapse.PLoS One. 2013 Jul 10;8(7):e68203. doi: 10.1371/journal.pone.0068203. Print 2013. PLoS One. 2013. PMID: 23874541 Free PMC article.
-
Hepatitis B Virus DNA Polymerase Displays an Anti-Apoptotic Effect by Interacting with Elongation Factor-1 Alpha-2 in Hepatoma Cells.J Microbiol Biotechnol. 2021 Jan 28;31(1):16-24. doi: 10.4014/jmb.2002.02039. J Microbiol Biotechnol. 2021. PMID: 33144545 Free PMC article.
-
Anti-HBV efficacy of combined siRNAs targeting viral gene and heat shock cognate 70.Virol J. 2012 Nov 16;9:275. doi: 10.1186/1743-422X-9-275. Virol J. 2012. PMID: 23158906 Free PMC article.
-
Hepatitis B virus X protein downregulates expression of the miR-16 family in malignant hepatocytes in vitro.Br J Cancer. 2011 Jun 28;105(1):146-53. doi: 10.1038/bjc.2011.190. Epub 2011 May 31. Br J Cancer. 2011. PMID: 21629246 Free PMC article.
-
Carboxyl-terminal truncated HBx contributes to invasion and metastasis via deregulating metastasis suppressors in hepatocellular carcinoma.Oncotarget. 2016 Aug 23;7(34):55110-55127. doi: 10.18632/oncotarget.10399. Oncotarget. 2016. PMID: 27391153 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

