Effects of human T-cell leukemia virus type 1 (HTLV-1) p13 on mitochondrial K+ permeability: A new member of the viroporin family?
- PMID: 20170654
- PMCID: PMC7163934
- DOI: 10.1016/j.febslet.2010.02.030
Effects of human T-cell leukemia virus type 1 (HTLV-1) p13 on mitochondrial K+ permeability: A new member of the viroporin family?
Abstract
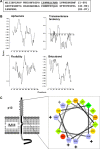
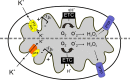
Human T-cell leukemia virus type-1 (HTLV-1) encodes a mitochondrial protein named p13. p13 mediates an inward K(+) current in isolated mitochondria that leads to mitochondrial swelling, depolarization, increased respiratory chain activity and reactive oxygen species (ROS) production. These effects trigger the opening of the permeability transition pore and are dependent on the presence of K(+) and on the amphipathic alpha helical domain of p13. In the context of cells, p13 acts as a sensitizer to selected apoptotic stimuli. Although it is not known whether p13 influences the activity of endogenous K(+) channels or forms a channel itself, it shares some structural and functional analogies with viroporins, a class of small integral membrane proteins that form pores and alter membrane permeability.
Copyright 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Modulation of mitochondrial K(+) permeability and reactive oxygen species production by the p13 protein of human T-cell leukemia virus type 1.Biochim Biophys Acta. 2009 Jul;1787(7):947-54. doi: 10.1016/j.bbabio.2009.02.001. Epub 2009 Feb 11. Biochim Biophys Acta. 2009. PMID: 19366603
-
The p13 protein of human T cell leukemia virus type 1 (HTLV-1) modulates mitochondrial membrane potential and calcium uptake.Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):945-51. doi: 10.1016/j.bbabio.2010.02.023. Epub 2010 Feb 25. Biochim Biophys Acta. 2010. PMID: 20188695
-
Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues.J Biol Chem. 2002 Sep 13;277(37):34424-33. doi: 10.1074/jbc.M203023200. Epub 2002 Jul 1. J Biol Chem. 2002. PMID: 12093802
-
The human T-cell leukemia virus type 1 p13II protein: effects on mitochondrial function and cell growth.Cell Death Differ. 2005 Aug;12 Suppl 1(Suppl 1):905-15. doi: 10.1038/sj.cdd.4401576. Cell Death Differ. 2005. PMID: 15761473 Free PMC article. Review.
-
Functional properties and sequence variation of HTLV-1 p13.Retrovirology. 2020 May 12;17(1):11. doi: 10.1186/s12977-020-00517-1. Retrovirology. 2020. PMID: 32398094 Free PMC article. Review.
Cited by
-
HTLV-1 Rex: the courier of viral messages making use of the host vehicle.Front Microbiol. 2012 Sep 6;3:330. doi: 10.3389/fmicb.2012.00330. eCollection 2012. Front Microbiol. 2012. PMID: 22973269 Free PMC article.
-
Hijacking the T-cell communication network by the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) p12 and p8 proteins.Mol Aspects Med. 2010 Oct;31(5):333-43. doi: 10.1016/j.mam.2010.07.001. Epub 2010 Jul 29. Mol Aspects Med. 2010. PMID: 20673780 Free PMC article. Review.
-
Mitochondrial Proteins Coded by Human Tumor Viruses.Front Microbiol. 2018 Feb 6;9:81. doi: 10.3389/fmicb.2018.00081. eCollection 2018. Front Microbiol. 2018. PMID: 29467726 Free PMC article. Review.
-
Localization of HPV-18 E2 at mitochondrial membranes induces ROS release and modulates host cell metabolism.PLoS One. 2013 Sep 24;8(9):e75625. doi: 10.1371/journal.pone.0075625. eCollection 2013. PLoS One. 2013. PMID: 24086592 Free PMC article.
-
Viroporins: structure and biological functions.Nat Rev Microbiol. 2012 Jul 2;10(8):563-74. doi: 10.1038/nrmicro2820. Nat Rev Microbiol. 2012. PMID: 22751485 Free PMC article. Review.
References
-
- Saggioro D., Silic-Benussi M., Biasiotto R., D’Agostino D.M., Ciminale V. Control of cell death pathways by HTLV-1 proteins. Front. Biosci. 2009;14:3338–3351. - PubMed
-
- Nicot C., Harrod R.L., Ciminale V., Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24:6026–6034. - PubMed
-
- D’Agostino D.M., Bernardi P., Chieco-Bianchi L., Ciminale V. Mitochondria as functional targets of proteins coded by human tumor viruses. Adv. Cancer Res. 2005;94:87–142. - PubMed
-
- D’Agostino D.M., Ciminale V., Pavlakis G.N., Chieco-Bianchi L. Intracellular trafficking of the human immunodeficiency virus type 1 Rev protein: involvement of continued rRNA synthesis in nuclear retention. AIDS Res. Hum. Retroviruses. 1995;11:1063–1071. - PubMed
-
- Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. ProteiN IDENTIFICATION AND ANALYSIS TOOLS On the ExPASy Server. The Proteomics Protocols Handbook. Humana Press; 2005. pp. 571–607.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

