Nervous-tissue-specific elimination of microtubule-actin crosslinking factor 1a results in multiple developmental defects in the mouse brain
- PMID: 20170731
- PMCID: PMC2847646
- DOI: 10.1016/j.mcn.2010.01.010
Nervous-tissue-specific elimination of microtubule-actin crosslinking factor 1a results in multiple developmental defects in the mouse brain
Abstract
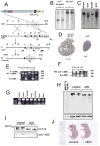
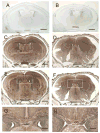
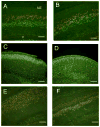
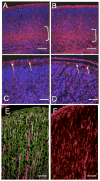
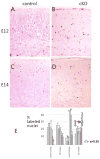
The microtubule-actin crosslinking factor 1 (MACF1) is a ubiquitous cytoskeletal linker protein with multiple spliced isoforms expressed in different tissues. The MACF1a isoform contains microtubule and actin-binding regions and is expressed at high levels in the nervous system. Macf1-/- mice are early embryonic lethal and hence the role of MACF1 in the nervous system could not be determined. We have specifically knocked out MACF1a in the developing mouse nervous system using Cre/loxP technology. Mutant mice died within 24-36h after birth of apparent respiratory distress. Their brains displayed a disorganized cerebral cortex with a mixed layer structure, heterotopia in the pyramidal layer of the hippocampus, disorganized thalamocortical and corticofugal fibers, and aplastic anterior and hippocampal commissures. Embryonic neurons showed a defect in traversing the cortical plate. Our data suggest a critical role for MACF1 in neuronal migration that is dependent on its ability to interact with both microfilaments and microtubules.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Blanchi B, Kelly LM, Viemari JC, Lafon I, Burnet H, Bevengut M, Tillmanns S, Daniel L, Graf T, Hilaire G, Sieweke MH. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci. 2003;6:1091–1100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

