Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents
- PMID: 20170749
- PMCID: PMC2854028
- DOI: 10.1016/j.cbpc.2010.02.005
Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents
Abstract
Ceruloplasmin (Cp) is a multicopper oxidase and the most abundant copper binding protein in vertebrate plasma. Loss of function mutations in humans or experimental deletion in mice result in iron overload consistent with a putative ferroxidase function. Prior work suggested plasma may contain multiple ferroxidases. Studies were conducted in Holtzman rats (Rattusnorvegicus), albino mice (Mus musculus), Cp-/- mice, and adult humans (Homo sapiens) to investigate the copper-iron interaction. Dietary copper-deficient (CuD) rats and mice were produced using a modified AIN-76A diet. Results confirmed that o-dianisidine is a better substrate than paraphenylene diamine (PPD) for assessing diamine oxidase activity of Cp. Plasma from CuD rat dams and pups, and CuD and Cp-/- mice contained no detectable Cp diamine oxidase activity. Importantly, no ferroxidase activity was detectable for CuD rats, mice, or Cp-/- mice compared to robust activity for copper-adequate (CuA) rodent controls using western membrane assay. Immunoblot protocols detected major reductions (60-90%) in Cp protein in plasma of CuD rodents but no alteration in liver mRNA levels by qRT-PCR. Data are consistent with apo-Cp being less stable than holo-Cp. Further research is needed to explain normal plasma iron in CuD mice. Reduction in Cp is a sensitive biomarker for copper deficiency.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

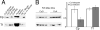
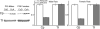
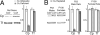
References
-
- Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J. Nutr. 2006;136:1236–1241. - PubMed
-
- Cherukuri S, Tripoulas NA, Nurko S, Fox PL. Anemia and impaired stress-induced erythropoiesis in aceruloplasminemic mice. Blood Cells Mol. Dis. 2004;33:346–355. - PubMed
-
- Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals. 2003;16:9–40. - PubMed
-
- Frieden E, Hsieh HS. Ceruloplasmin: the copper transport protein with essential oxidase activity. Adv Enzymol Rel Areas Mol Biol. 1976;44:187–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

