Important elements for the diagnosis of drug-induced liver injury
- PMID: 20170750
- PMCID: PMC3901223
- DOI: 10.1016/j.cgh.2010.02.008
Important elements for the diagnosis of drug-induced liver injury
Abstract
Background & aims: Drug-induced liver disease is the leading cause of acute liver failure in the United States. Accurate reporting of drug-induced liver injury is essential for early detection of hepatotoxicity and for developing reliable, interpretable literature. We assessed the extent to which published case reports of drug-induced liver disease include sufficient clinical data for interpreting the cause of toxicity.
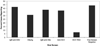
Methods: We developed a list of 42 predetermined, specific minimal elements necessary in evaluating causality of drug-induced liver injury. We then analyzed 97 published case reports or series studies of hepatotoxicity from 6 drugs (from 3 classes): amoxicillin/clavulanic acid (n = 35), troglitazone (n = 32), rosiglitazone (n = 10), pioglitazone (n = 8), zafirlukast (n = 8), and montelukast (n = 4).
Results: Patient age, sex, primary disease, and drug name were reported in most, if not all, published case reports. However, many elements were underreported; some publications did not mention initial bilirubin levels (12%), many did not provide initial alkaline phosphatase levels (58%), and others provided vague descriptions of how certain diagnoses were excluded, that is, tests for hepatitis A, B, and C were negative. Data on abnormal results from serial liver tests frequently were absent. Exclusions of competing viral etiologies were reported in less than 50% of the studies.
Conclusions: Reports of drug-induced liver diseases often do not provide the data needed to determine the causes of the adverse effects. Efforts to promote and include a list of essential diagnostic elements in research articles could increase the quality and clinical utility of published case reports of drug toxicity.
Copyright (c) 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Galan MV, Potts JA, Silverman AL, et al. The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center. J Clin Gastroenterol. 2005;39:64–67. - PubMed
-
- Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. - PubMed
-
- Venulet J. Informativity of adverse reactions data in medical publications. Drug Inf J. 1985;19:357–365.
-
- Kelly WN. The quality of published adverse drug event reports. Ann Pharmacother. 2003;37:1774–1778. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 DK065211/DK/NIDDK NIH HHS/United States
- 1U01DK065238/DK/NIDDK NIH HHS/United States
- U01 DK083027/DK/NIDDK NIH HHS/United States
- U01 DK083020/DK/NIDDK NIH HHS/United States
- U01 DK065201/DK/NIDDK NIH HHS/United States
- U01 DK065238/DK/NIDDK NIH HHS/United States
- U01 DK065193/DK/NIDDK NIH HHS/United States
- 1U01DK065201/DK/NIDDK NIH HHS/United States
- U01 DK065176/DK/NIDDK NIH HHS/United States
- U01 DK083023/DK/NIDDK NIH HHS/United States
- 1U01DK065176/DK/NIDDK NIH HHS/United States
- U01 DK082992/DK/NIDDK NIH HHS/United States
- 1U01DK065193/DK/NIDDK NIH HHS/United States
- 1U01DK065211/DK/NIDDK NIH HHS/United States
- 1U01DK065184/DK/NIDDK NIH HHS/United States
- U01 DK065184/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

