FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a
- PMID: 20171206
- PMCID: PMC2854211
- DOI: 10.1016/j.ydbio.2010.02.016
FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a
Abstract
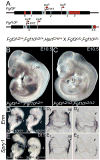
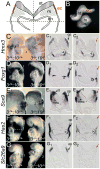
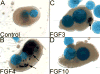
The inner ear epithelium, with its complex array of sensory, non-sensory, and neuronal cell types necessary for hearing and balance, is derived from a thickened patch of head ectoderm called the otic placode. Mouse embryos lacking both Fgf3 and Fgf10 fail to initiate inner ear development because appropriate patterns of gene expression fail to be specified within the pre-otic field. To understand the transcriptional "blueprint" initiating inner ear development, we used microarray analysis to identify prospective placode genes that were differentially expressed in control and Fgf3(-)(/)(-);Fgf10(-)(/)(-) embryos. Several genes in the down-regulated class, including Hmx3, Hmx2, Foxg1, Sox9, Has2, and Slc26a9 were validated by in situ hybridization. We also assayed candidate target genes suggested by other studies of otic induction. Two placode markers, Fgf4 and Foxi3, were down-regulated in Fgf3(-)(/)(-);Fgf10(-)(/)(-) embryos, whereas Foxi2, a cranial epidermis marker, was expanded in double mutants, similar to its behavior when WNT responses are blocked in the otic placode. Assays of hindbrain Wnt genes revealed that only Wnt8a was reduced or absent in FGF-deficient embryos, and that even some Fgf3(-)(/)(-);Fgf10(-)(/+) and Fgf3(-)(/)(-) embryos failed to express Wnt8a, suggesting a key role for Fgf3, and a secondary role for Fgf10, in Wnt8a expression. Chick explant assays showed that FGF3 or FGF4, but not FGF10, were sufficient to induce Wnt8a. Collectively, our results suggest that Wnt8a provides the link between FGF-induced formation of the pre-otic field and restriction of the otic placode to ectoderm adjacent to the hindbrain.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Roles of Wnt8a during formation and patterning of the mouse inner ear.Mech Dev. 2013 Feb;130(2-3):160-8. doi: 10.1016/j.mod.2012.09.009. Epub 2012 Oct 4. Mech Dev. 2013. PMID: 23041177
-
Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning.Mech Dev. 2002 Nov;119(1):91-108. doi: 10.1016/s0925-4773(02)00343-x. Mech Dev. 2002. PMID: 12385757
-
Fgf3 and Fgf10 are required for mouse otic placode induction.Development. 2003 Aug;130(15):3379-90. doi: 10.1242/dev.00555. Development. 2003. PMID: 12810586
-
The first steps towards hearing: mechanisms of otic placode induction.Int J Dev Biol. 2007;51(6-7):463-72. doi: 10.1387/ijdb.072320to. Int J Dev Biol. 2007. PMID: 17891709 Review.
-
Changing shape and shaping change: Inducing the inner ear.Semin Cell Dev Biol. 2017 May;65:39-46. doi: 10.1016/j.semcdb.2016.10.006. Epub 2016 Oct 29. Semin Cell Dev Biol. 2017. PMID: 27989562 Review.
Cited by
-
A more efficient method to generate null mutants using Hprt-Cre with floxed alleles.J Pediatr Surg. 2011 Sep;46(9):1711-9. doi: 10.1016/j.jpedsurg.2011.01.023. J Pediatr Surg. 2011. PMID: 21929979 Free PMC article.
-
A gene network regulated by FGF signalling during ear development.Sci Rep. 2017 Jul 21;7(1):6162. doi: 10.1038/s41598-017-05472-0. Sci Rep. 2017. PMID: 28733657 Free PMC article.
-
Mesodermal Fgf10b cooperates with other fibroblast growth factors during induction of otic and epibranchial placodes in zebrafish.Dev Dyn. 2014 Oct;243(10):1275-85. doi: 10.1002/dvdy.24119. Epub 2014 Mar 3. Dev Dyn. 2014. PMID: 24677486 Free PMC article.
-
Early Steps towards Hearing: Placodes and Sensory Development.Int J Mol Sci. 2023 Apr 10;24(8):6994. doi: 10.3390/ijms24086994. Int J Mol Sci. 2023. PMID: 37108158 Free PMC article. Review.
-
Regeneration of Hair Cells: Making Sense of All the Noise.Pharmaceuticals (Basel). 2011 Jun 1;4(6):848-879. doi: 10.3390/ph4060848. Pharmaceuticals (Basel). 2011. PMID: 21966254 Free PMC article.
References
-
- Adamska M, Leger S, Brand M, Hadrys T, Braun T, Bober E. Inner ear and lateral line expression of a zebrafish Nkx5-1 gene and its downregulation in the ears of FGF8 mutant, ace. Mech Dev. 2000;97:161–5. - PubMed
-
- Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–38. - PubMed
-
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–77. - PubMed
-
- Barrionuevo F, Naumann A, Bagheri-Fam S, Speth V, Taketo MM, Scherer G, Neubuser A. Sox9 is required for invagination of the otic placode in mice. Dev Biol. 2008;317:213–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

