Arsenic trioxide and ascorbic acid demonstrate promising activity against primary human CLL cells in vitro
- PMID: 20171736
- PMCID: PMC4164821
- DOI: 10.1016/j.leukres.2010.01.020
Arsenic trioxide and ascorbic acid demonstrate promising activity against primary human CLL cells in vitro
Abstract
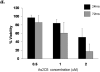
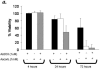
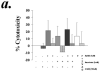
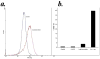
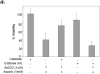
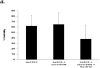
The compromised antioxidant defense system in chronic lymphocytic leukemia (CLL) suggested a potential use for reactive oxygen species (ROS) generating arsenic trioxide (ATO) and ascorbic acid. While both ATO and ascorbic acid mediate cytotoxicity in CLL B cells as single agents, the efficacy of ATO is enhanced by ascorbic acid. This effect is dependent on increased ROS accumulation, as pretreatment of B-CLL cells with a glutathione reducing buthionine sulfoximine or catalase inhibiting aminotriazole, enhanced ATO/ascorbic acid-mediated cytotoxicity. Pretreatment with reducing agents such as catalase, or thiol antioxidant, N-acetyl cysteine or GSH also abrogated ATO/ascorbic acid-mediated cytotoxicity. Furthermore, Hu1D10-mediated cell death was enhanced with ATO and ascorbic acid, thus justifying potential combination of ATO/arsenic trioxide therapy with antibodies such as Hu1D10 that also cause accumulation of ROS.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures







Similar articles
-
Arsenic trioxide induces human pulmonary fibroblast cell death via increasing ROS levels and GSH depletion.Oncol Rep. 2012 Aug;28(2):749-57. doi: 10.3892/or.2012.1852. Epub 2012 Jun 6. Oncol Rep. 2012. PMID: 22684917
-
Induction of apoptosis in arsenic trioxide-treated lung cancer A549 cells by buthionine sulfoximine.Mol Cells. 2008 Aug 31;26(2):158-64. Epub 2008 Jul 3. Mol Cells. 2008. PMID: 18596414
-
Differential augmentative effects of buthionine sulfoximine and ascorbic acid in As2O3-induced ovarian cancer cell death: oxidative stress-independent and -dependent cytotoxic potentiation.Int J Oncol. 2011 Jun;38(6):1731-9. doi: 10.3892/ijo.2011.986. Epub 2011 Mar 23. Int J Oncol. 2011. PMID: 21455570
-
Arsenic trioxide: mechanisms of action.Semin Hematol. 2002 Apr;39(2 Suppl 1):3-7. doi: 10.1053/shem.2002.33610. Semin Hematol. 2002. PMID: 12012315 Review.
-
Combination therapy with arsenic trioxide for hematological malignancies.Anticancer Agents Med Chem. 2010 Jul;10(6):504-10. doi: 10.2174/1871520611009060504. Anticancer Agents Med Chem. 2010. PMID: 20812901 Review.
Cited by
-
Synthesis and Evaluation of Folate-Conjugated Phenanthraquinones for Tumor-Targeted Oxidative Chemotherapy.Open J Med Chem. 2016 Mar;6(1):1-17. doi: 10.4236/ojmc.2016.61001. Epub 2016 Mar 11. Open J Med Chem. 2016. PMID: 27066312 Free PMC article.
-
Arsenic trioxide induces apoptosis in B-cell chronic lymphocytic leukemic cells through down-regulation of survivin via the p53-dependent signaling pathway.Leuk Res. 2013 Dec;37(12):1719-25. doi: 10.1016/j.leukres.2013.09.019. Epub 2013 Sep 29. Leuk Res. 2013. PMID: 24211095 Free PMC article.
-
Ascorbic acid: chemistry, biology and the treatment of cancer.Biochim Biophys Acta. 2012 Dec;1826(2):443-57. doi: 10.1016/j.bbcan.2012.06.003. Epub 2012 Jun 20. Biochim Biophys Acta. 2012. PMID: 22728050 Free PMC article. Review.
-
Ascorbic Acid Sensitizes Colorectal Carcinoma to the Cytotoxicity of Arsenic Trioxide via Promoting Reactive Oxygen Species-Dependent Apoptosis and Pyroptosis.Front Pharmacol. 2020 Feb 21;11:123. doi: 10.3389/fphar.2020.00123. eCollection 2020. Front Pharmacol. 2020. PMID: 32153415 Free PMC article.
-
Organometallic nucleosides induce non-classical leukemic cell death that is mitochondrial-ROS dependent and facilitated by TCL1-oncogene burden.Mol Cancer. 2015 Jun 4;14:114. doi: 10.1186/s12943-015-0378-1. Mol Cancer. 2015. PMID: 26041471 Free PMC article.
References
-
- Farber CM, Liebes LF, Kanganis DN, Silber R. Human B lymphocytes show greater susceptibility to H2O2 toxicity than T lymphocytes. J Immunol. 1984;132:2543–2546. - PubMed
-
- Oltra AM, Carbonell F, Tormos C, Iradi A, Saez GT. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free Radic Biol Med. 2001;30:1286–1292. - PubMed
-
- Farber CM, Kanganis DN, Liebes LF, Silber R. Antioxidant enzymes in lymphocytes from normal subjects and patients with chronic lymphocytic leukaemia: increased glutathione peroxidase activity in CLL B lymphocytes. Br J Haematol. 1989;72:32–35. - PubMed
-
- Moran EC, Kamiguti AS, Cawley JC, Pettitt AR. Cytoprotective antioxidant activity of serum albumin and autocrine catalase in chronic lymphocytic leukaemia. Br J Haematol. 2002;116:316–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

