Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS)
- PMID: 20171748
- PMCID: PMC4028132
- DOI: 10.1016/j.aquatox.2010.02.003
Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS)
Abstract
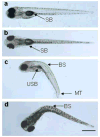
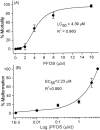
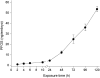
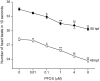
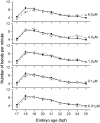
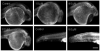
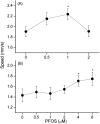
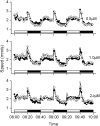
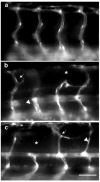
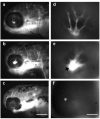
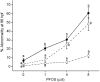
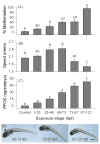
Perfluorooctanesulphonicacid (PFOS), a persistent organic contaminant, has been widely detected in the environment, wildlife and humans, but few studies have assessed its effect on aquatic organisms. The present study evaluated the effect of PFOS on zebrafish embryos. Zebrafish embryos exhibited developmental toxicity of bent spine, uninflated swim bladder, decreased heart rate and affected spontaneous movement after exposure to various PFOS concentrations (0-8mg/L) from 6 to 120h post-fertilization (hpf). The LC(50) at 120hpf was 2.20mg/L and the EC(50) at 120hpf was 1.12mg/L. Continuous exposure to PFOS from 1 to 121hpf resulted in a steady accumulation with no evidence of elimination. PFOS induced cell death at 24hpf was consistently found in the brain, eye, and tail region of embryos. PFOS exposure induced lesions in the muscle fibers with histological examination. Behavior assessment of PFOS in zebrafish embryos elevated the basal rate of swimming after 4 days of exposure, and larvae exposed to PFOS (0.25-4mg/L) for only 1h at 6dpf swam faster with increasing PFOS concentration. Embryos/larvae exposed to 8mg/L PFOS for 24h periods from 1 to 121hpf showed the highest incidence of malformations in the 97-121hpf window. This is the first study to define uptake kinetics and to focus on behavioral consequences following PFOS exposure in zebrafish. Our results further the understanding of the toxicity of PFOS to aquatic organisms and suggest the need for additional research to identify the mode of PFOS toxicity.
Figures
Similar articles
-
6:2 Chlorinated polyfluorinated ether sulfonate, a PFOS alternative, induces embryotoxicity and disrupts cardiac development in zebrafish embryos.Aquat Toxicol. 2017 Apr;185:67-75. doi: 10.1016/j.aquatox.2017.02.002. Epub 2017 Feb 3. Aquat Toxicol. 2017. PMID: 28187362
-
PFOS affects posterior swim bladder chamber inflation and swimming performance of zebrafish larvae.Aquat Toxicol. 2014 Dec;157:225-35. doi: 10.1016/j.aquatox.2014.10.017. Epub 2014 Oct 27. Aquat Toxicol. 2014. PMID: 25456237
-
Early life perfluorooctanesulphonic acid (PFOS) exposure impairs zebrafish organogenesis.Aquat Toxicol. 2014 May;150:124-32. doi: 10.1016/j.aquatox.2014.03.005. Epub 2014 Mar 12. Aquat Toxicol. 2014. PMID: 24667235 Free PMC article.
-
The presence of MWCNTs reduces developmental toxicity of PFOS in early life stage of zebrafish.Environ Pollut. 2017 Mar;222:201-209. doi: 10.1016/j.envpol.2016.12.055. Epub 2017 Jan 4. Environ Pollut. 2017. PMID: 28063710
-
6:2 fluorotelomer sulfonamide alkylbetaine (6:2 FTAB), a novel perfluorooctane sulfonate alternative, induced developmental toxicity in zebrafish embryos.Aquat Toxicol. 2018 Feb;195:24-32. doi: 10.1016/j.aquatox.2017.12.002. Epub 2017 Dec 7. Aquat Toxicol. 2018. PMID: 29247975
Cited by
-
Embryonic exposure to PFAS causes long-term, compound-specific behavioral alterations in zebrafish.Neurotoxicol Teratol. 2023 May-Jun;97:107165. doi: 10.1016/j.ntt.2023.107165. Epub 2023 Feb 18. Neurotoxicol Teratol. 2023. PMID: 36801483 Free PMC article.
-
Comparison of waterborne and in ovo nanoinjection exposures to assess effects of PFOS on zebrafish embryos.Environ Sci Pollut Res Int. 2015 Feb;22(3):2303-10. doi: 10.1007/s11356-014-3527-y. Epub 2014 Sep 4. Environ Sci Pollut Res Int. 2015. PMID: 25182431
-
Small fish making a big difference: beloved star of environmental toxicology research in the current era.J Zhejiang Univ Sci B. 2025 Jul 15;26(7):613-632. doi: 10.1631/jzus.B2500166. J Zhejiang Univ Sci B. 2025. PMID: 40722242 Free PMC article. Review.
-
Short-term exposure of zebrafish embryos to arecoline leads to retarded growth, motor impairment, and somite muscle fiber changes.Zebrafish. 2015 Feb;12(1):58-70. doi: 10.1089/zeb.2014.1010. Epub 2014 Dec 30. Zebrafish. 2015. PMID: 25549301 Free PMC article.
-
Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring.Environ Toxicol Chem. 2011 Sep;30(9):2073-80. doi: 10.1002/etc.594. Epub 2011 Jun 30. Environ Toxicol Chem. 2011. PMID: 21671259 Free PMC article.
References
-
- Ahmadi FA, Linseman DA, Grammatopoulos TN, Jones SM, Bouchard RJ, Freed CR, Heidenreich KA, Zawada WM. The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J Neurochem. 2003;87:914–921. - PubMed
-
- Butenhoff J, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, Jung R, Kennedy G, Jr, Lieder P, Olsen G, Thomford P. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkey after oral dosing for 6 months. Toxicol Sci. 2002;69:244–257. - PubMed
-
- Cheng SH, Wai AWK, So CH, Wu RSS. Cellular and molecular basis of cadmium-induced deformities in zebrafish embryos. Environ Toxicol Chem. 2000;19:3024–3031.
-
- Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Dev Biol. 2001;240:123–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

